Abstract
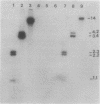
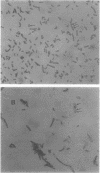
The poly-beta-hydroxybutyrate (PHB) biosynthetic pathway from Alcaligenes eutrophus H16 has been cloned and expressed in Escherichia coli. Initially, an A. eutrophus H16 genomic library was constructed by using cosmid pVK102, and cosmid clones that encoded the PHB biosynthetic pathway were sought by assaying for the first enzyme of the pathway, beta-ketothiolase. Six enzyme-positive clones were identified. Three of these clones manifested acetoacetyl coenzyme A reductase activity, the second enzyme of the biosynthetic pathway, and accumulated PHB. PHB was produced in the cosmid clones at approximately 50% of the level found in A. eutrophus. One cosmid clone was subjected to subcloning experiments, and the PHB biosynthetic pathway was isolated on a 5.2-kilobase KpnI-EcoRI fragment. This fragment, when cloned into small multicopy vectors, can direct the synthesis of PHB in E. coli to levels approaching 80% of the bacterial cell dry weight.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K., Wilke-Douglas M. Construction and use of a gene bank of Alcaligenes eutrophus in the analysis of ribulose bisphosphate carboxylase genes. J Bacteriol. 1984 Sep;159(3):973–978. doi: 10.1128/jb.159.3.973-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. A method for the ligation of DNA following isolation from low melting temperature agarose. Anal Biochem. 1983 Nov;135(1):48–51. doi: 10.1016/0003-2697(83)90728-5. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Castillo R., Rodriguez-Valera F., Gonzalez-Ramos J., Ruiz-Berraquero F. Accumulation of Poly (beta-Hydroxybutyrate) by Halobacteria. Appl Environ Microbiol. 1986 Jan;51(1):214–216. doi: 10.1128/aem.51.1.214-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- Griebel R. J., Merrick J. M. Metabolism of poly- -hydroxybutyrate: effect of mild alkaline extraction on native poly- -hydroxybutyrate granules. J Bacteriol. 1971 Nov;108(2):782–789. doi: 10.1128/jb.108.2.782-789.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F. A., Dawes E. A. Regulation of the tricarboxylic acid cycle and poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii grown under nitrogen or oxygen limitation. J Gen Microbiol. 1976 Dec;97(2):303–312. doi: 10.1099/00221287-97-2-303. [DOI] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Oeding V., Schlegel H. G. Beta-ketothiolase from Hydrogenomonas eutropha H16 and its significance in the regulation of poly-beta-hydroxybutyrate metabolism. Biochem J. 1973 May;134(1):239–248. doi: 10.1042/bj1340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Masamune S., Walsh C. T., Sinskey A. J. Biosynthetic thiolase from Zoogloea ramigera. III. Isolation and characterization of the structural gene. J Biol Chem. 1987 Jan 5;262(1):97–102. [PubMed] [Google Scholar]
- Repaske R., Repaske A. C. Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):585–591. doi: 10.1128/aem.32.4.585-591.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. Poly- -hydroxybutyrate biosynthesis and the regulation of glucose metabolism in Azotobacter beijerinckii. Biochem J. 1971 Nov;125(1):55–66. doi: 10.1042/bj1250055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J. 1973 May;134(1):225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakisaka Y., Masaki E., Nishimoto Y. Formation of Crystalline delta-Endotoxin or Poly-beta-Hydroxybutyric Acid Granules by Asporogenous Mutants of Bacillus thuringiensis. Appl Environ Microbiol. 1982 Jun;43(6):1473–1480. doi: 10.1128/aem.43.6.1473-1480.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. C., Dawes E. A. A disk assay for poly- -hydroxybutyrate. Anal Biochem. 1973 Apr;52(2):607–613. doi: 10.1016/0003-2697(73)90067-5. [DOI] [PubMed] [Google Scholar]