Abstract
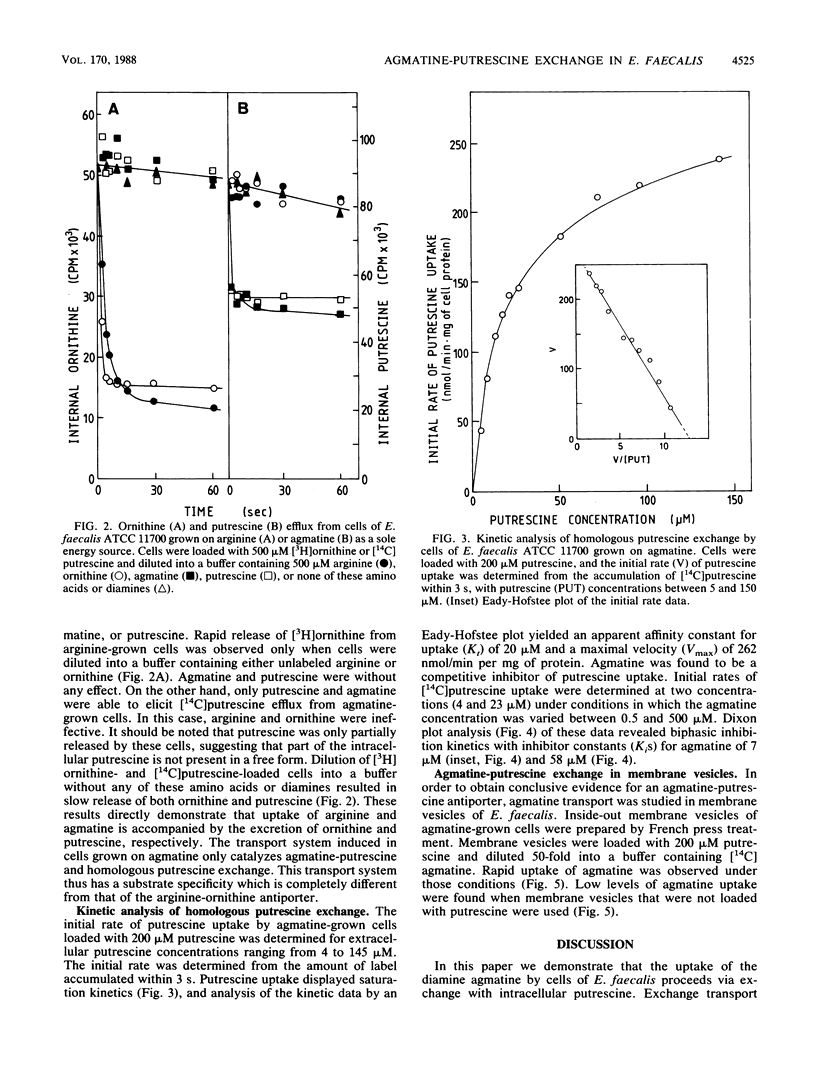
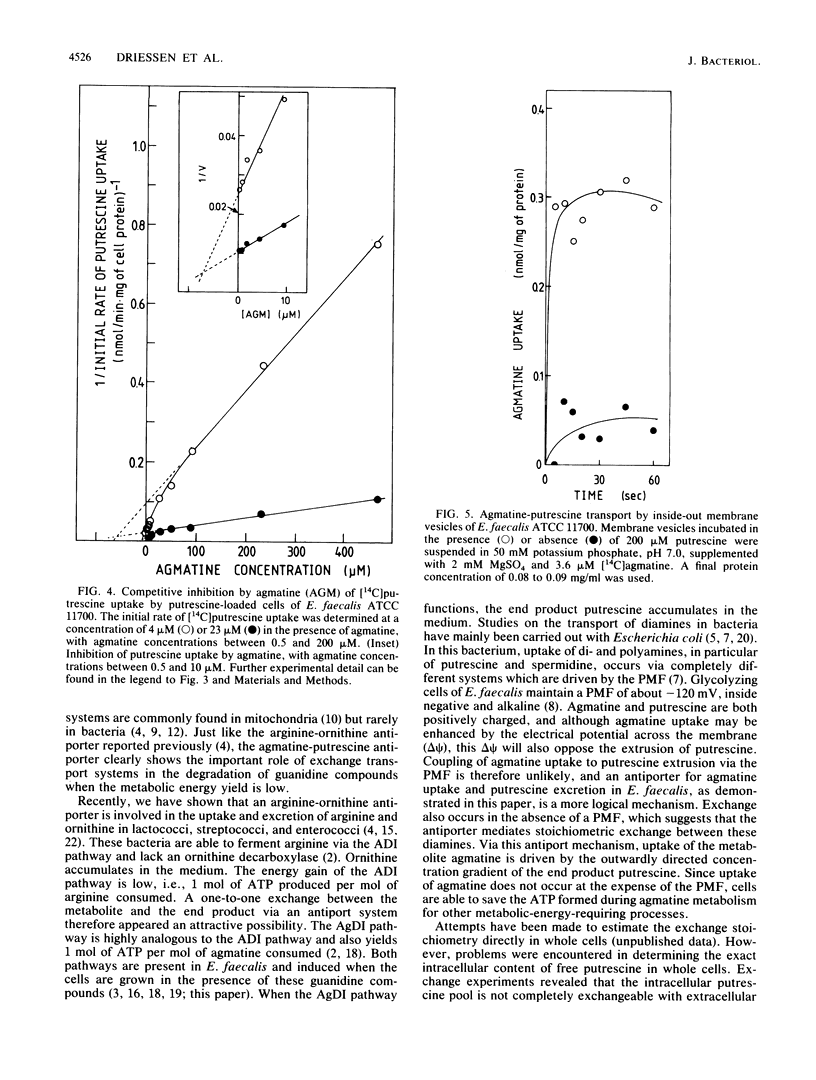
Enterococcus faecalis ATCC 11700 is able to use arginine and the diamine agmatine as a sole energy source. Via the highly homologous deiminase pathways, arginine and agmatine are converted into CO2, NH3, and the end products ornithine and putrescine, respectively. In the arginine deiminase pathway, uptake of arginine and excretion of ornithine are mediated by an arginine-ornithine antiport system. The translocation of agmatine was studied in whole cells grown in the presence of arginine, agmatine, or glucose. Rapid uncoupler-insensitive uptake of agmatine was observed only in agmatine-grown cells. A high intracellular putrescine pool was maintained by these cells, and this pool was rapidly released by external putrescine or agmatine but not by arginine or ornithine. Kinetic analysis revealed competitive inhibition for uptake between putrescine and agmatine. Agmatine uptake by membrane vesicles was observed only when the membrane vesicles were preloaded with putrescine. Uptake of agmatine was driven by the outwardly directed putrescine concentration gradient, which is continuously sustained by the metabolic process. Uptake of agmatine and extrusion of putrescine by agmatine-grown cells of E. faecalis appeared to be catalyzed by an agmatine-putrescine antiporter. This transport system functionally resembled the previously described arginine-ornithine antiport, which was exclusively induced when the cells were grown in the presence of arginine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crow V. L., Thomas T. D. Arginine metabolism in lactic streptococci. J Bacteriol. 1982 Jun;150(3):1024–1032. doi: 10.1128/jb.150.3.1024-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel R. H. Utilization of arginine as an energy source for the growth of Streptococcus faecalis. J Bacteriol. 1964 May;87(5):988–992. doi: 10.1128/jb.87.5.988-992.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Poolman B., Kiewiet R., Konings W. Arginine transport in Streptococcus lactis is catalyzed by a cationic exchanger. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6093–6097. doi: 10.1073/pnas.84.17.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houng H. S., Lynn A. R., Rosen B. P. ATP-driven calcium transport in membrane vesicles of Streptococcus sanguis. J Bacteriol. 1986 Nov;168(2):1040–1044. doi: 10.1128/jb.168.2.1040-1044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V. Streptomycin uptake via an inducible polyamine transport system in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):345–351. doi: 10.1111/j.1432-1033.1978.tb12316.x. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K., Kobayashi H., Igarashi K. Apparently unidirectional polyamine transport by proton motive force in polyamine-deficient Escherichia coli. J Bacteriol. 1986 Mar;165(3):972–977. doi: 10.1128/jb.165.3.972-977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Murakami N., Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [PubMed] [Google Scholar]
- Krause D. C., Winkler H. H., Wood D. O. Cloning and expression of the Rickettsia prowazekii ADP/ATP translocator in Escherichia coli. Proc Natl Acad Sci U S A. 1985 May;82(9):3015–3019. doi: 10.1073/pnas.82.9.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Boeker E. A. Biosynthetic and biodegradative ornithine and arginine decarboxylases from Escherichia coli. Methods Enzymol. 1983;94:125–134. doi: 10.1016/s0076-6879(83)94020-x. [DOI] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987 Dec;169(12):5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Barker H. A. Fermentation of agmatine in Streptococcus faecalis: occurrence of putrescine transcarbamoylase. J Bacteriol. 1972 Jan;109(1):44–50. doi: 10.1128/jb.109.1.44-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. P., Stalon V. Enzymes of agmatine degradation and the control of their synthesis in Streptococcus faecalis. J Bacteriol. 1982 Nov;152(2):676–681. doi: 10.1128/jb.152.2.676-681.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. P., Wargnies B., Stalon V. Control of enzyme synthesis in the arginine deiminase pathway of Streptococcus faecalis. J Bacteriol. 1982 Jun;150(3):1085–1090. doi: 10.1128/jb.150.3.1085-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Ornithine transport and exchange in Streptococcus lactis. J Bacteriol. 1987 Sep;169(9):4147–4153. doi: 10.1128/jb.169.9.4147-4153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargnies B., Lauwers N., Stalon V. Structure and properties of the putrescine carbamoyltransferase of Streptococcus faecalis. Eur J Biochem. 1979 Nov 1;101(1):143–152. doi: 10.1111/j.1432-1033.1979.tb04226.x. [DOI] [PubMed] [Google Scholar]



