Abstract
Expression of the Saccharomyces cerevisiae PIS gene encoding phosphatidylinositol synthase in Escherichia coli was achieved by inserting its coding sequence into lacZ on pUC8. The fused gene encoded a phosphatidylinositol synthase whose amino-terminal three amino acids had been replaced by the amino-terminal five amino acids of E. coli beta-galactosidase. E. coli cells bearing this recombinant plasmid produced a significant level of phosphatidylinositol synthase in the presence of a lacZ inducer, isopropylthio-beta-D-galactopyranoside. When the culture medium was supplemented with myo-inositol and isopropylthio-beta-D-galactopyranoside, the cells accumulated a substantial amount of phosphatidylinositol in their membranes. When a saturating level of myo-inositol was added, phosphatidylinositol constituted about 4% of the total phospholipids. Phosphatidylinositol accumulation occurred at the expense of phosphatidylglycerol. The ratio of phosphatidylethanolamine to total acidic phospholipids remained constant. The growth rate of phosphatidylinositol-containing E. coli cells did not differ significantly from that of cells with the normal phospholipid composition.
Full text
PDF

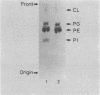
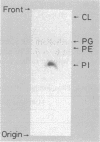
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLOU C. E., VILKAS E., LEDERER E. Structural studies on the myo-inositol phospholipids of Mycobacterium tuberculosis (var. bovis, strain BCG). J Biol Chem. 1963 Jan;238:69–76. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- HUSTON C. K., ALBRO P. W., GRINDEY G. B. LIPIDS OF SARCINA LUTEA. 3. COMPOSITION OF THE COMPLEX LIPIDS. J Bacteriol. 1965 Mar;89:768–775. doi: 10.1128/jb.89.3.768-775.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Brody S. Glycerophospholipid variation in choline and inositol auxotrophs of Neurospora crassa. Internal compensation among zwitterionic and anionic species. J Biol Chem. 1975 Sep 25;250(18):7173–7181. [PubMed] [Google Scholar]
- Ikawa M. Bacterial phosphatides and natural relationships. Bacteriol Rev. 1967 Mar;31(1):54–64. doi: 10.1128/br.31.1.54-64.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Miyajima A., Arai K., Matsumoto K. Possible involvement of RAS-encoded proteins in glucose-induced inositolphospholipid turnover in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8172–8176. doi: 10.1073/pnas.83.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Lester R. L., Steiner M. R. The occurrence of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. J Biol Chem. 1968 Sep 25;243(18):4889–4893. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nikawa J., Kodaki T., Yamashita S. Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J Biol Chem. 1987 Apr 5;262(10):4876–4881. [PubMed] [Google Scholar]
- Nikawa J., Nagumo T., Yamashita S. Myo-inositol transport in Saccharomyces cerevisiae. J Bacteriol. 1982 May;150(2):441–446. doi: 10.1128/jb.150.2.441-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J., Tsukagoshi Y., Kodaki T., Yamashita S. Nucleotide sequence and characterization of the yeast PSS gene encoding phosphatidylserine synthase. Eur J Biochem. 1987 Aug 17;167(1):7–12. doi: 10.1111/j.1432-1033.1987.tb13297.x. [DOI] [PubMed] [Google Scholar]
- Nikawa J., Yamashita S. Molecular cloning of the gene encoding CDPdiacylglycerol-inositol 3-phosphatidyl transferase in Saccharomyces cerevisiae. Eur J Biochem. 1984 Sep 3;143(2):251–256. doi: 10.1111/j.1432-1033.1984.tb08366.x. [DOI] [PubMed] [Google Scholar]
- Nikawa J., Yamashita S. Yeast mutant defective in synthesis of phosphatidylinositol. Isolation and characterization of a CDPdiacylglycerol--inositol 3-phosphatidyltransferase Km mutant. Eur J Biochem. 1982 Jul;125(2):445–451. doi: 10.1111/j.1432-1033.1982.tb06703.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Larson T. J., Dowhan W. Gene cloning for the isolation of enzymes of membrane lipid synthesis: phosphatidylserine synthase overproduction in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1412–1416. doi: 10.1073/pnas.74.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]