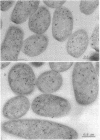
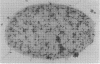
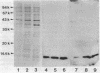
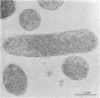
Abstract
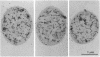
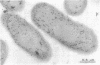
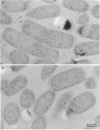
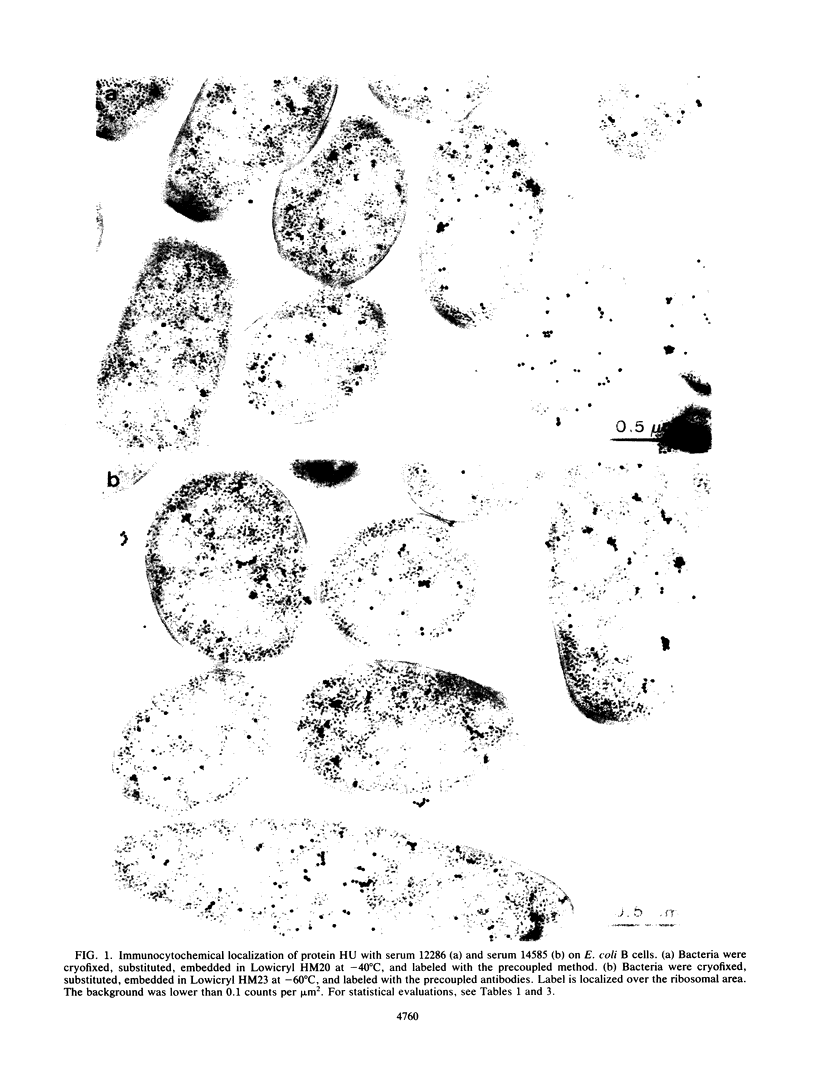
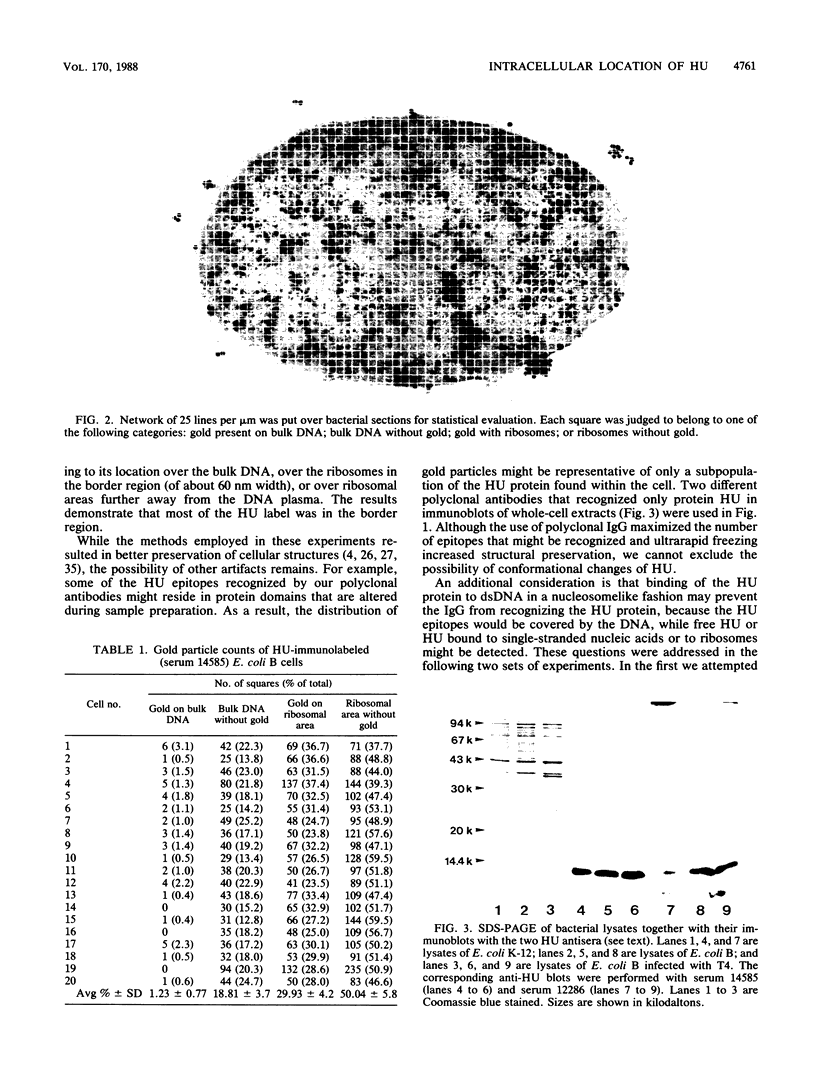
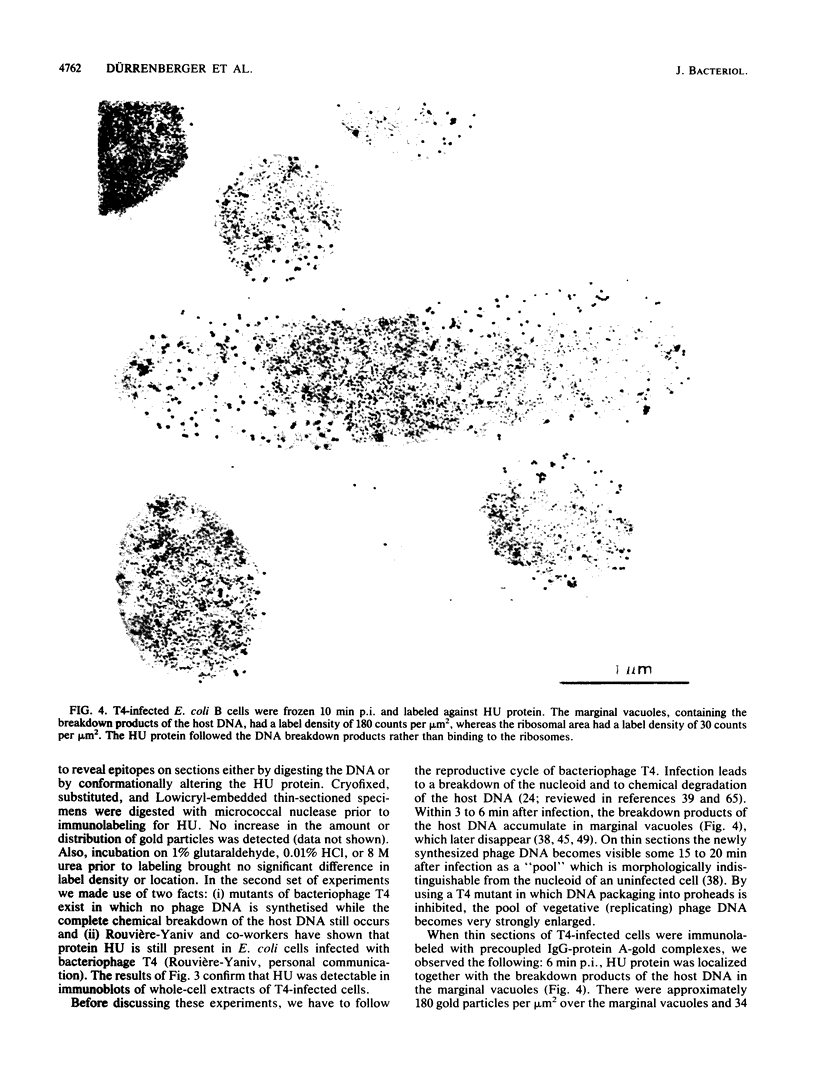
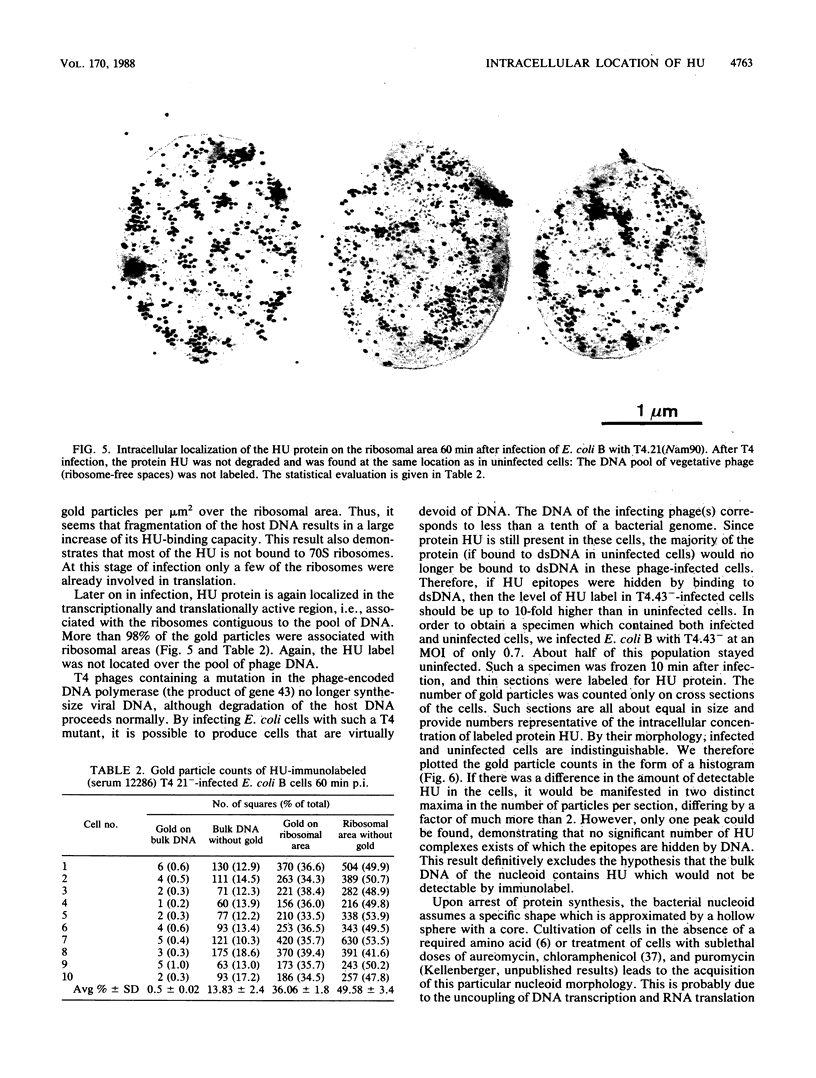
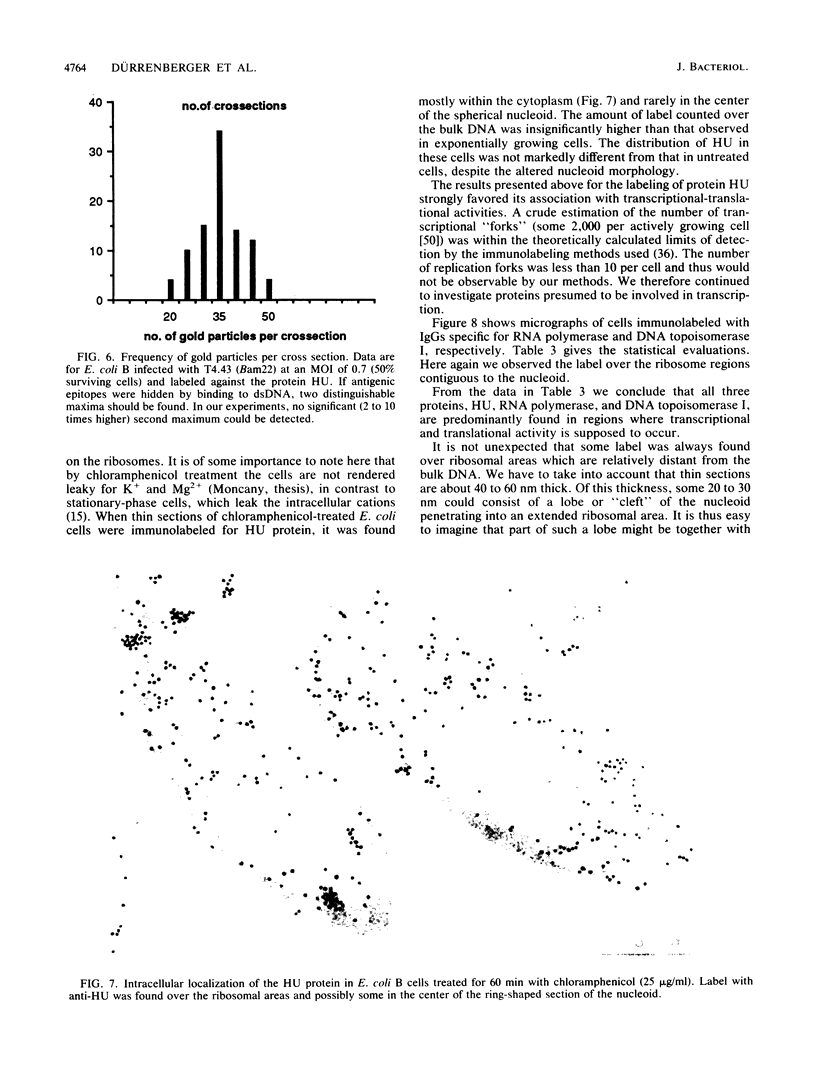
Immunocytochemical labeling of thin sections of cryosubstituted, Lowicryl-embedded Escherichia coli cells with protein A-colloidal gold was used to study the structural organization of the bacterial nucleoid. We found that the histonelike protein HU was not associated with the bulk DNA in the nucleoid but was located in areas of the cell where metabolically active DNA is associated with ribosomes and where single-stranded DNA, RNA polymerase, and DNA topoisomerase I were also located. The resolution of the methods used did not allow us to decide whether HU was associated either with ribosomes or with transcriptionally active DNA, nor could we demonstrate interaction of HU with either.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Berthold V., Geider K. Interaction of DNA with DNA-binding proteins. The characterization of protein HD from Escherichia coli and its nucleic acid complexes. Eur J Biochem. 1976 Dec 11;71(2):443–449. doi: 10.1111/j.1432-1033.1976.tb11132.x. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Chai N. C., Lark K. G. Cytological studies of deoxyribonucleic acid replication in Escherichia coli 15T-: replication at slow growth rates and after a shift-up into rich medium. J Bacteriol. 1970 Oct;104(1):401–409. doi: 10.1128/jb.104.1.401-409.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R., Arndt-Jovin D. J., Mizuuchi K. A defined system for the DNA strand-transfer reaction at the initiation of bacteriophage Mu transposition: protein and DNA substrate requirements. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7570–7574. doi: 10.1073/pnas.82.22.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire K. M., Grindley N. D. Replicative and conservative transposition in bacteria. Cell. 1986 Nov 7;47(3):325–327. doi: 10.1016/0092-8674(86)90586-6. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Kornberg A. Protein HU in the enzymatic replication of the chromosomal origin of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):424–428. doi: 10.1073/pnas.81.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., McDowall A. W., Menge B., Schmid E. N., Lickfeld K. G. Electron microscopy of frozen-hydrated bacteria. J Bacteriol. 1983 Jul;155(1):381–390. doi: 10.1128/jb.155.1.381-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978 Feb;13(2):295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. The organisation of chromatin loops: characterization of a scaffold attachment site. EMBO J. 1986 Mar;5(3):511–518. doi: 10.1002/j.1460-2075.1986.tb04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K. Interaction of DNA with DNA-binding proteins: protein exchange and complex stability. Eur J Biochem. 1978 Jul 3;87(3):617–622. doi: 10.1111/j.1432-1033.1978.tb12414.x. [DOI] [PubMed] [Google Scholar]
- Greene J. R., Brennan S. M., Andrew D. J., Thompson C. C., Richards S. H., Heinrikson R. L., Geiduschek E. P. Sequence of the bacteriophage SP01 gene coding for transcription factor 1, a viral homologue of the bacterial type II DNA-binding proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7031–7035. doi: 10.1073/pnas.81.22.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Visualization of prokaryotic DNA in a regularly condensed chromatin-like fiber. Proc Natl Acad Sci U S A. 1976 Feb;73(2):563–567. doi: 10.1073/pnas.73.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., DIXON J., CHASE M. Nucleic acid economy in bacteria infected with bacteriophage T2. I. Purine and pyrimidine composition. J Gen Physiol. 1953 Jul;36(6):777–789. doi: 10.1085/jgp.36.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Chang M., Kim U. J., Grunstein M. Histone H2B repression causes cell-cycle-specific arrest in yeast: effects on chromosomal segregation, replication, and transcription. Cell. 1987 Feb 27;48(4):589–597. doi: 10.1016/0092-8674(87)90237-6. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Bjornsti M. A., Kellenberger E. Use of on-section immunolabeling and cryosubstitution for studies of bacterial DNA distribution. J Bacteriol. 1987 May;169(5):2055–2062. doi: 10.1128/jb.169.5.2055-2062.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Lutz H., Kornberg A. Novel histone H2A-like protein of escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5097–5101. doi: 10.1073/pnas.77.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber R., Bächinger H., Bickle T. A. Purification and characterisation of a small DNA-binding protein, HB, from Bacillus globigii. Eur J Biochem. 1982 Mar 1;122(3):627–632. doi: 10.1111/j.1432-1033.1982.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Jehl B., Bauer R., Dörge A., Rick R. The use of propane/isopentane mixtures for rapid freezing of biological specimens. J Microsc. 1981 Sep;123(Pt 3):307–309. doi: 10.1111/j.1365-2818.1981.tb02475.x. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. About the organisation of condensed and decondensed non-eukaryotic DNA and the concept of vegetative DNA (a critical review). Biophys Chem. 1988 Feb;29(1-2):51–62. doi: 10.1016/0301-4622(88)87024-8. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Carlemalm E., Stauffer E., Kellenberger C., Wunderli H. In vitro studies of the fixation of DNA, nucleoprotamine, nucleohistone and proteins. Eur J Cell Biol. 1981 Aug;25(1):1–4. [PubMed] [Google Scholar]
- Kellenberger E., Dürrenberger M., Villiger W., Carlemalm E., Wurtz M. The efficiency of immunolabel on Lowicryl sections compared to theoretical predictions. J Histochem Cytochem. 1987 Sep;35(9):959–969. doi: 10.1177/35.9.3302020. [DOI] [PubMed] [Google Scholar]
- Koerner J. F., Snustad D. P. Shutoff of host macromolecular synthesis after T-even bacteriophage infection. Microbiol Rev. 1979 Jun;43(2):199–223. doi: 10.1128/mr.43.2.199-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. Chromatin staining of bacteria during bacteriophage infection. J Bacteriol. 1950 Apr;59(4):551–560. doi: 10.1128/jb.59.4.551-560.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine B., Kmiecik D., Sautiere P., Biserte G., Cohen-Solal M. Complete amino-acid sequences of DNA-binding proteins HU-1 and HU-2 from Escherichia coli. Eur J Biochem. 1980 Feb;103(3):447–461. doi: 10.1111/j.1432-1033.1980.tb05968.x. [DOI] [PubMed] [Google Scholar]
- Laine B., Sautiere P., Spassky A., Rimsky S. A DNA-binding protein from E. coli isolation, characterization and its relationship with proteins H1 and B1. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1147–1153. doi: 10.1016/0006-291x(84)90895-7. [DOI] [PubMed] [Google Scholar]
- Lathe R., Buc H., Lecocq J. P., Bautz E. K. Prokaryotic histone-like protein interacting with RNA polymerase. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3548–3552. doi: 10.1073/pnas.77.6.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch Y., LaPointe J. W., Kornberg R. D. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell. 1987 Apr 24;49(2):203–210. doi: 10.1016/0092-8674(87)90561-7. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G. E., GILLEN D. H., HEAGY F. C. Cytological changes in Escherichia coli produced by infection with phage T2. J Bacteriol. 1950 May;59(5):603–615. doi: 10.1128/jb.59.5.603-615.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende L., Timm B., Subramanian R. Primary structures of two homologous ribosome-associated DNA-binding proteins of Escherichia coli. FEBS Lett. 1978 Dec 15;96(2):395–398. doi: 10.1016/0014-5793(78)80446-3. [DOI] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Structure and properties of the bacterial nucleoid. Cell. 1982 Oct;30(3):667–669. doi: 10.1016/0092-8674(82)90269-0. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Manes S. H., Drlica K. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell. 1982 Nov;31(1):35–42. doi: 10.1016/0092-8674(82)90402-0. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Reichlin M., Nisonoff A., Margoliash E. Immunological activity of cytochrome c. 3. Enhancement of antibody detection and immune response initiation by cytochrome c polymers. J Biol Chem. 1970 Mar 10;245(5):947–954. [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Kjeldgaard N. O. Native Escherichia coli HU protein is a heterotypic dimer. FEBS Lett. 1979 Oct 15;106(2):297–300. doi: 10.1016/0014-5793(79)80518-9. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J. Localization of the HU protein on the Escherichia coli nucleoid. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):439–447. doi: 10.1101/sqb.1978.042.01.047. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Ryter A., Chang A. Localization of transcribing genes in the bacterial cell by means of high resolution autoradiography. J Mol Biol. 1975 Nov 15;98(4):797–810. doi: 10.1016/s0022-2836(75)80011-8. [DOI] [PubMed] [Google Scholar]
- Saavedra R. A., Huberman J. A. Both DNA topoisomerases I and II relax 2 micron plasmid DNA in living yeast cells. Cell. 1986 Apr 11;45(1):65–70. doi: 10.1016/0092-8674(86)90538-6. [DOI] [PubMed] [Google Scholar]
- Salti V., Le Hégarat F., Hirschbein L. Isolation and characterization of small heat-stable acid-soluble DNA-binding proteins from Bacillus subtilis nucleoids. J Gen Microbiol. 1985 Mar;131(3):581–590. doi: 10.1099/00221287-131-3-581. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Pettijohn D. E. Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci U S A. 1981 Jan;78(1):224–228. doi: 10.1073/pnas.78.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayana T., Subramanian A. R. Specific association of two homologous DNA-binding proteins to the native 30-S ribosomal subunits of Escherichia coli. Biochim Biophys Acta. 1978 Sep 27;520(2):342–357. doi: 10.1016/0005-2787(78)90232-0. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Nedospasov S. A., Georgiev G. P. On the structure of eukaryotic, prokaryotic, and viral chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):457–473. doi: 10.1101/sqb.1978.042.01.049. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Nagata A., Kano Y., Imamoto F. Isolation and characterization of nucleoid proteins from Escherichia coli. Mol Gen Genet. 1984;196(2):217–224. doi: 10.1007/BF00328053. [DOI] [PubMed] [Google Scholar]