Abstract
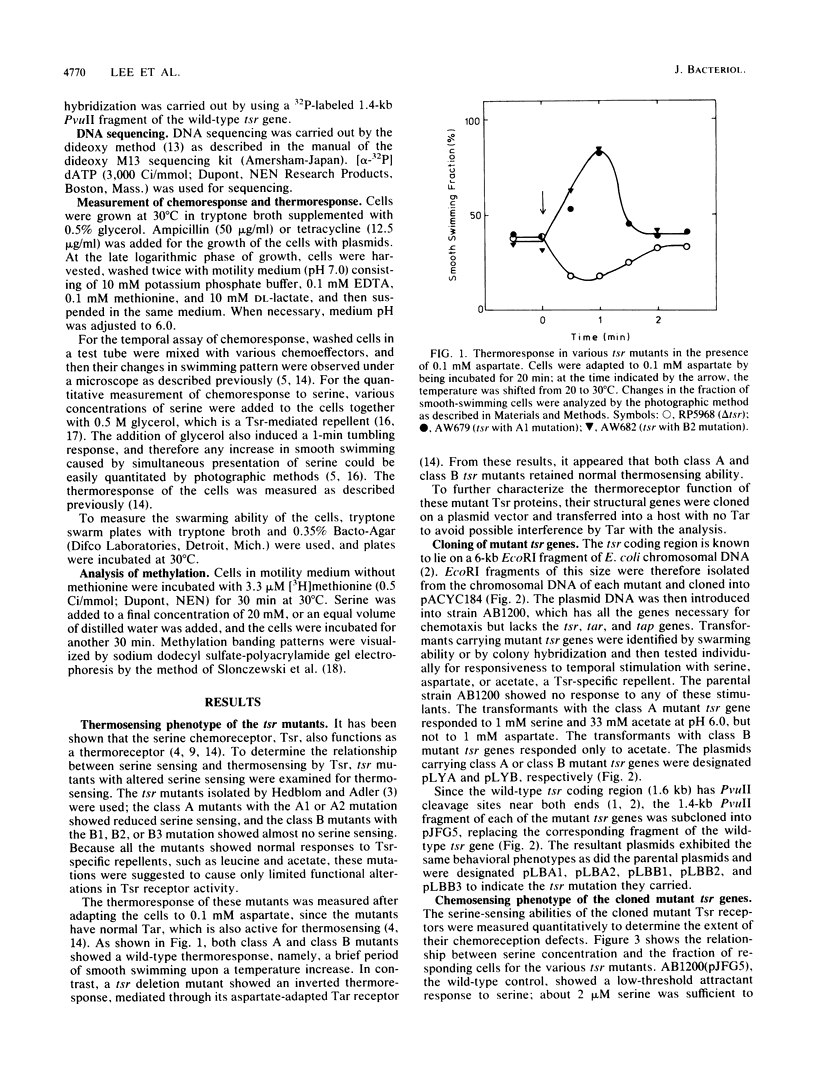
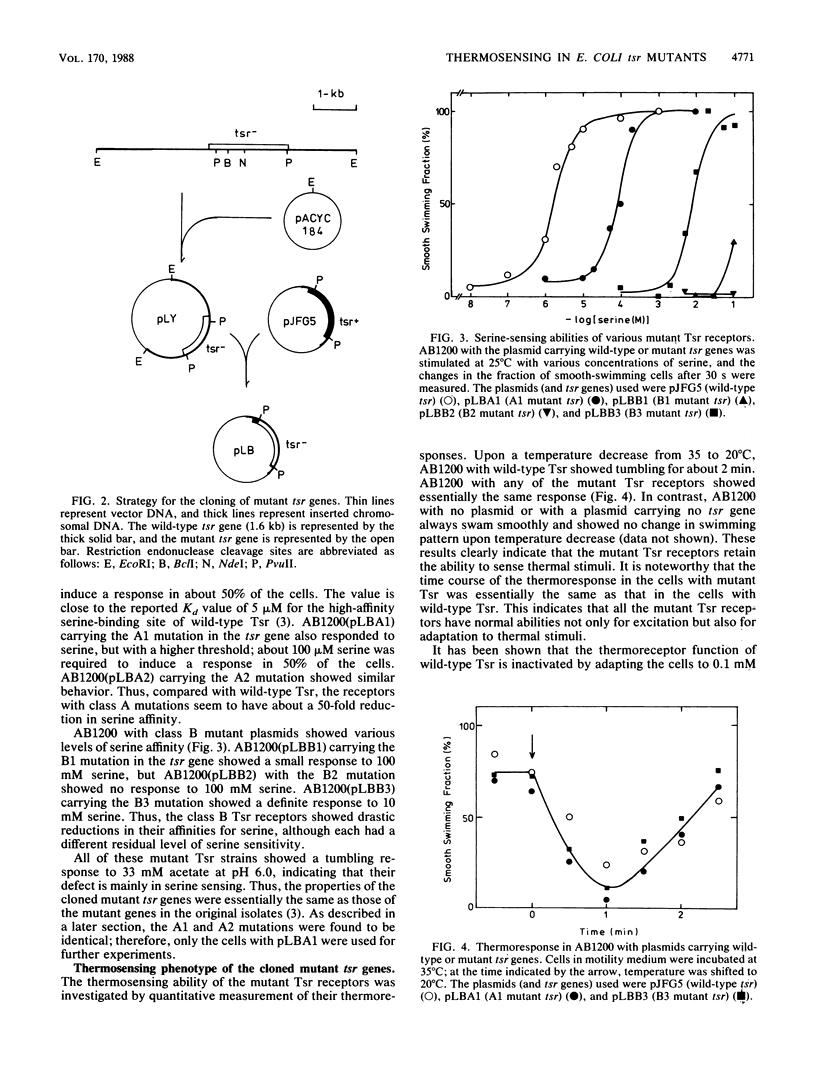
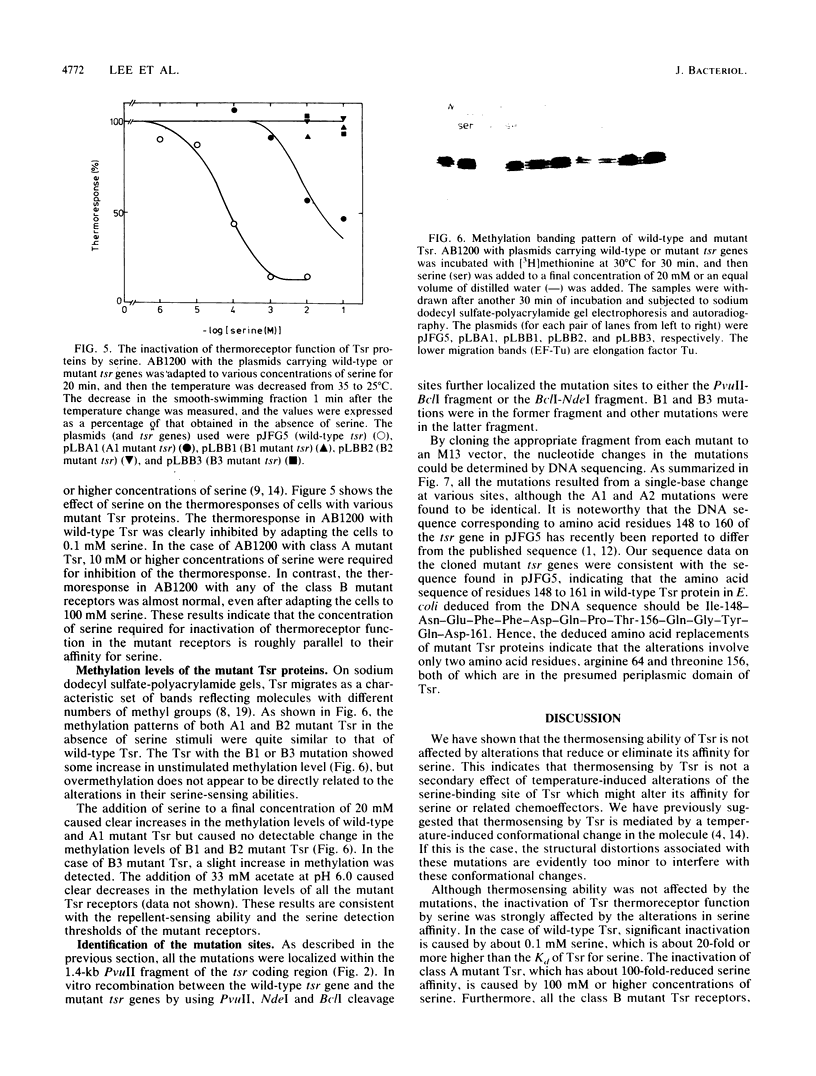
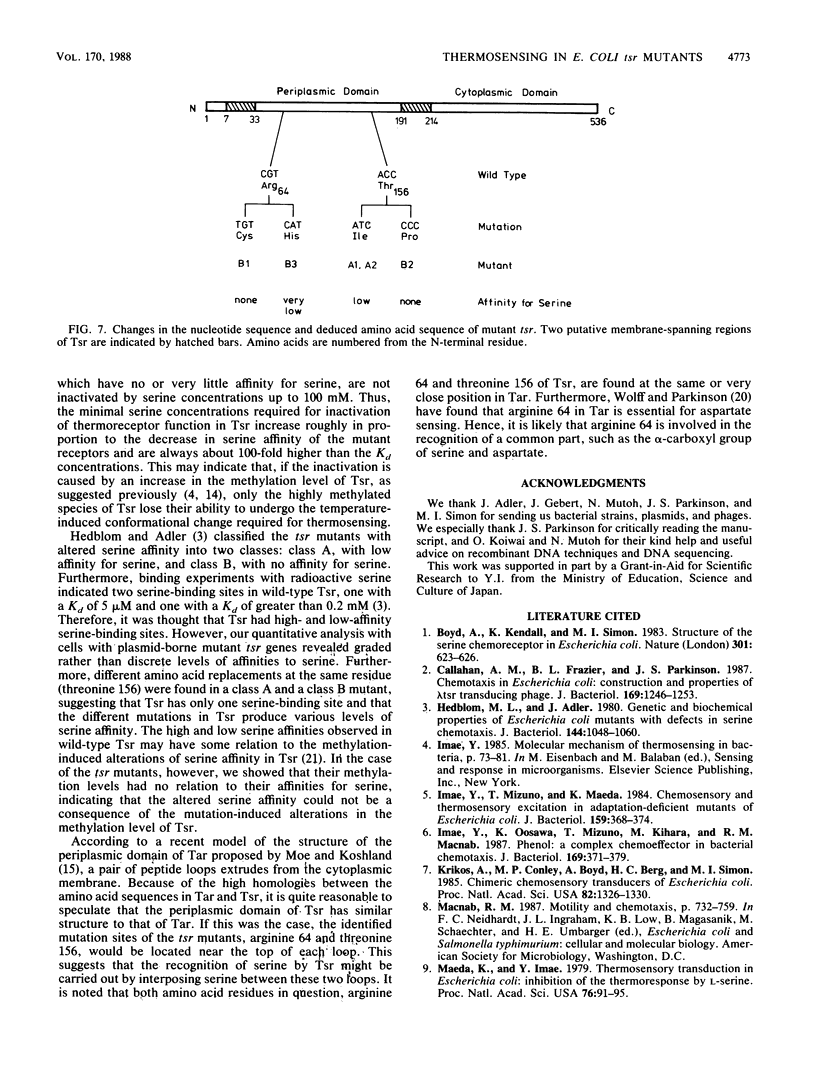
Tsr, a chemoreceptor for serine and repellents in Escherichia coli, also functions as a thermoreceptor. The relationship between the chemoreceptor and thermoreceptor functions of Tsr was examined in five tsr mutants with altered serine detection thresholds. The thermosensing abilities of the mutant Tsr proteins were not affected by the alterations in their affinities to serine. In contrast, the ability of serine to inactivate thermoreceptor function was altered in these mutants. The minimal serine concentration required for thermoreceptor inactivation was directly related to the decreased affinity of the mutant Tsr for serine. The amino acid replacements in the mutant receptors were deduced from DNA sequence analyses and occurred at two different locations in the presumed periplasmic domain of Tsr. Two mutations caused histidine or cysteine replacements at arginine 64, whereas three others caused isoleucine or proline replacements at threonine 156.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd A., Kendall K., Simon M. I. Structure of the serine chemoreceptor in Escherichia coli. Nature. 1983 Feb 17;301(5901):623–626. doi: 10.1038/301623a0. [DOI] [PubMed] [Google Scholar]
- Callahan A. M., Frazier B. L., Parkinson J. S. Chemotaxis in Escherichia coli: construction and properties of lambda tsr transducing phage. J Bacteriol. 1987 Mar;169(3):1246–1253. doi: 10.1128/jb.169.3.1246-1253.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedblom M. L., Adler J. Genetic and biochemical properties of Escherichia coli mutants with defects in serine chemotaxis. J Bacteriol. 1980 Dec;144(3):1048–1060. doi: 10.1128/jb.144.3.1048-1060.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imae Y., Mizuno T., Maeda K. Chemosensory and thermosensory excitation in adaptation-deficient mutants of Escherichia coli. J Bacteriol. 1984 Jul;159(1):368–374. doi: 10.1128/jb.159.1.368-374.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imae Y., Oosawa K., Mizuno T., Kihara M., Macnab R. M. Phenol: a complex chemoeffector in bacterial chemotaxis. J Bacteriol. 1987 Jan;169(1):371–379. doi: 10.1128/jb.169.1.371-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikos A., Conley M. P., Boyd A., Berg H. C., Simon M. I. Chimeric chemosensory transducers of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1326–1330. doi: 10.1073/pnas.82.5.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Imae Y., Shioi J. I., Oosawa F. Effect of temperature on motility and chemotaxis of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1039–1046. doi: 10.1128/jb.127.3.1039-1046.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Imae Y. Thermosensory transduction in Escherichia coli: inhibition of the thermoresponse by L-serine. Proc Natl Acad Sci U S A. 1979 Jan;76(1):91–95. doi: 10.1073/pnas.76.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Imae Y. Conditional inversion of the thermoresponse in Escherichia coli. J Bacteriol. 1984 Jul;159(1):360–367. doi: 10.1128/jb.159.1.360-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Imae Y. Demethylation of methyl-accepting chemotaxis proteins in Escherichia coli induced by the repellents glycerol and ethylene glycol. J Bacteriol. 1984 Feb;157(2):576–581. doi: 10.1128/jb.157.2.576-581.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Imae Y. Glycerol and ethylene glycol: members of a new class of repellents of Escherichia coli chemotaxis. J Bacteriol. 1983 Apr;154(1):104–112. doi: 10.1128/jb.154.1.104-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Macnab R. M., Alger J. R., Castle A. M. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli. J Bacteriol. 1982 Oct;152(1):384–399. doi: 10.1128/jb.152.1.384-399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C., Parkinson J. S. Aspartate taxis mutants of the Escherichia coli tar chemoreceptor. J Bacteriol. 1988 Oct;170(10):4509–4515. doi: 10.1128/jb.170.10.4509-4515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa H., Hayashi H. Desensitization by covalent modification of the chemoreceptor of Escherichia coli. FEBS Lett. 1986 Mar 17;198(1):21–24. doi: 10.1016/0014-5793(86)81176-0. [DOI] [PubMed] [Google Scholar]




