Abstract
The effector domain mutants of oncogenic Ras, V12S35 Ras, V12G37 Ras, and V12C40 Ras were tested for their abilities to mediate tumorigenic and metastatic phenotypes in athymic nude mice when expressed in NIH 3T3 fibroblasts. All mutants displayed comparable tumorigenic properties, but only the mutant that activates the Raf-mitogen-activated protein kinase kinase (MEK)-extracellular regulated kinase (ERK) 1/2 pathway, V12S35 Ras, induced tumors in the experimental metastasis assay. Furthermore, direct activation of the MEK-ERK1/2 pathway in NIH 3T3 cells by mos or a constitutively active form of MEK was sufficient to induce metastasis whereas R-Ras, which fails to activate the ERK1/2 pathway, is tumorigenic but nonmetastatic. The subcutaneous tumors and lung metastases derived from V12S35 Ras-transformed NIH 3T3 cells expressed higher levels of activated ERK1/2 in culture when compared with the parental cellular pool before injection, indicating that selection for cells with higher levels of activated ERK1/2 occurred during tumor growth and metastasis. By contrast, cells explanted from V12G37-Ras or V12C40-Ras-induced tumors did not show changes in the level of ERK1/2 activation when compared with the parental cells. When tumor-explanted cell lines derived from each of the effector domain mutants were passaged one additional time in vivo, all mediated rapid tumor growth, but, again, only cells derived from V12S35 Ras-tumors formed numerous metastatic lesions within the lung. These results show that the metastatic properties of the Ras effector domain mutants segregate, and that, whereas Ras-mediated tumorigenicity can arise independently of ERK1/2 activation, experimental metastasis appears to require constitutive activation of the ERK1/2 pathway.
The precise molecular events leading to the acquisition of the metastatic phenotype remain largely unclear. Members of the Ras super family of small GTP-binding proteins have been implicated in the regulation of diverse biological functions, including tumor progression. Activating mutations in ras genes are commonly found in a variety of human tumors, and, in addition, oncogenic mutants of Ras confer both tumorigenic and metastatic properties to rodent cells in culture, providing a system for determining the molecular events necessary for tumor progression (1). In its active GTP-bound state, Ras activates a number of signaling pathways through its ability to activate key effector proteins. The most extensively studied of these is the mitogen-activated protein (MAP) kinase 1/2 pathway (2). Ras associates with and activates the serine kinase Raf-1, which in turn phosphorylates the dual specific kinase MEK (also known as MAP kinase kinase or MAPKK), which in turn phosphorylates the MAP kinases ERK1 and 2 (for extracellular regulated kinases 1 and 2). Activated MAP kinases translocate to the nucleus whereby they modulate gene expression (3, 4).
A large body of evidence suggests a central role for the Raf-MEK-ERK1/2 pathway in a variety of Ras-mediated responses, but more recent studies (2, 5) have implicated Raf-independent pathways in diverse biological events mediated by the Ras oncogene, including cytoskeletal reorganization, gene expression, and cell cycle progression. For example, members of the Rho family of small GTP-binding proteins have been shown to act as key regulators of Ras-mediated cytoskeletal modifications and proliferation (2, 6). In addition, the Rho GTPases have been implicated in motility and invasion (7–10), and it has been suggested that Rho may serve as an essential mediator during tumor metastasis (11). However, the precise Ras-mediated signaling pathways and downstream genetic events key to the acquisition of the metastatic phenotype remain to be elucidated.
A region of the Ras protein known as the effector domain (amino acids 32–40 in H-Ras) has been shown to be essential for the interaction between Ras and a variety of effectors, which in mammals include Raf-1, PI3-K (phosphatidylinositol 3-kinase), RasGAPs, Ral guanine nucleotide exchange factors, MEK kinase 1, Rin1 and AF6/Rsb1 (2, 12). Point mutations within the effector domain of oncogenic ras generates mutants deficient in specific effector function and therefore activation of specific downstream signaling pathways (13). By using these Ras effector domain mutants (V12S35 Ras, V12G37 Ras, and V12C40 Ras), it was shown that Ras-mediated tumorigenic transformation can occur independently of the Raf-MEK-ERK1/2 pathway (13, 14). Here, we show that, although both Raf-dependent and Raf-independent Ras signaling pathways mediate tumor growth, the Raf-MEK-ERK1/2 pathway appears necessary for activity in experimental metastasis assays. Thus, the Ras effector pathways mediating the tumorigenic and metastatic activities can be segregated.
METHODS
Molecular Constructs, Cell Lines, and Transfections.
All ras expression constructs encode mutant versions of the transforming human H-Ras (V12) protein. The pDCR-ras (V12), pDCR-ras (V12,S35), pDCR-ras (V12, G37), and pDCR-ras (V12, C40) mammalian constructs encode effector domain mutants of H-Ras (V12) in which expression is under the control of the cytomegalovirus promoter and in which the protein product is HA-tagged at the N terminus (13, 14). The R-Ras expression construct (V38 R-Ras) in the pMT-SM plasmid was a kind gift from Alan Hall (University College of London, United Kingdom). NIH 3T3 (490) cells were grown in DMEM supplemented with 10% fetal bovine serum and were maintained at 37°C in a humidified atmosphere of 10% CO2. NIH 3T3 cells transformed with the mouse mos oncogene (15) and constitutively activated MEK (16) have been described and were cultured as above.
DNA transfections were performed by using the DOTAP reagent as per manufacturers instructions (Boehringer Mannheim) by using 10 μg of DNA per 100 mm culture dish. After transfections, cells were placed under selection (400 μg/ml Geneticin), and multiple G418 resistant clones (>100) were pooled together to establish each cell line used in subsequent studies.
Western Blot Analysis and Immunoprecipitation.
Cells were treated as indicated and were lysed as described (17). Protein samples (10 μg) in Laemmli buffer were resolved by SDS/PAGE. After transfer to nitrocellulose, blots were probed with the following primary antibodies: rabbit polyclonal α-Ha (Y11, Santa Cruz Biotechnology); rabbit polyclonal α-Raf-1 (C12, Santa Cruz Biotechnology); rabbit polyclonal α-phospho-MEK1/2 (New England Biolabs); rabbit polyclonal α-MEK1/2 (Transduction Laboratories, Lexington, KY); rabbit polyclonal α-phospho-ERK1/2 (Promega); mouse monoclonal α-ERK1/2 (Zymed); rabbit polyclonal α-Met (SP 260, Santa Cruz Biotechnology); and mouse monoclonal α-phosphotyrosine (4G10, Upstate Biotechnology, Lake Placid, NY). After incubation with the appropriate peroxidase-conjugated secondary antibody (Boehringer Mannheim), proteins were visualized by using the enhanced chemiluminescence (ECL) system (Amersham).
Immunoprecipitations were performed as described (17) by using 500 μg of cell lysates incubated overnight at 4°C with the desired antibody (α-HA or α-Met). Resulting immune complexes then were pelleted by using protein-A-agarose (Sigma), were washed with lysis buffer, and were resuspended in Laemmli buffer. Samples then were subjected to Western blotting as described above.
In Vitro ERK1/2 Kinase Assay.
The method for detecting ERK1/2 activity in cells expressing the different Ras effector domain mutants was adapted from that of Khosravi-Far et al. (14). ERKs 1 and 2 were immunoprecipitated from cell lysates as described above by using a mouse monoclonal anti-ERK1/2 antibody (Zymed; clone ERK-7D8). Immune complexes were washed three times with lysis buffer and twice with kinase buffer (25 mM Hepes, pH 7.2/100 mM NaCl/5 mM MgCl2/1 mM DTT/0.1 mM sodium vanadate/10 μM ATP/0.1% BSA/0.1% Triton-X-100). Each kinase reaction was carried out in the presence of 4 μg myelin basic protein and 10 μCi of {γ-32P} ATP at 30°C for 30 minutes. The reactions were stopped by the addition of 1/3 volume of 4× Laemmli buffer. The proteins then were separated by 14% SDS/PAGE and were visualized by autoradiography.
In Vivo Tumorigenicity and Metastasis Assays and Histological Analysis.
The mice utilized in these experiments were ≈4-week-old female athymic nudes (Ncr nu/nu) that were obtained from the animal protection area, National Cancer Institute, Frederick Cancer Research and Development Center, Frederick MD. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86–23, 1985).
For the analysis of tumor growth and experimental metastasis, 105 cells (0.1 ml) were injected s.c. and i.v., respectively, into nude mice as described (18). Animals were killed when they appeared distressed or after 14 weeks. Tumor-derived cultures were obtained from s.c. grown tumors and lung metastases after killing the animals, dissecting and mincing the tumor tissue, and plating in DMEM/10% fetal bovine serum medium containing 400 μg/ml active Geneticin to select against growth of host tissue.
For histological examination, gross necropsies were performed on a representative selection of animals from each group. Tissues were fixed in 10% buffered neutral formalin at necropsy and were examined for gross lesions suggestive of metastasis. Tissues and lesions were processed in paraffin, were sectioned at 5 μm, and were stained with hematoxylin and eosin. An additional 50-μm step section was made on the lung block.
RESULTS
Characterization of Ras Effector Domain Mutants in NIH 3T3 (490) cells.
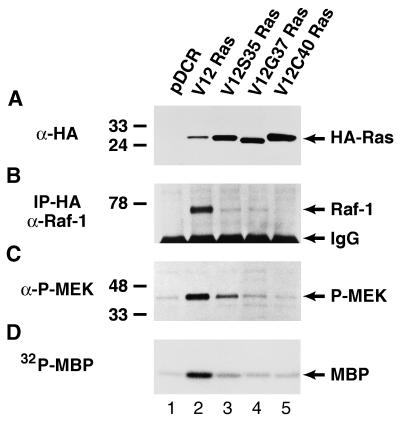
NIH 3T3 cells transfected with the various oncogenic Ras (V12) effector domain mutants were analyzed for expression of HA-tagged Ras proteins by Western blot analysis (Fig. 1A). The levels of expression were similar between the three effector domain mutants used in these studies (V12S35 Ras, V12G37 Ras, and V12C40 Ras; Fig. 1, lanes 3, 4, and 5, respectively) and were notably higher than that observed in cells transfected with the wild-type (wt) ras oncogene (wt V12 Ras; Fig. 1, lane 2). These Ras effector domain mutants were identified and characterized in part on the basis of their differential association with the Raf-1 cytoplasmic kinase (13, 14). We, therefore, determined the activity of the Raf-MEK-ERK1/2 pathway in NIH 3T3 (490) cells expressing the different Ras mutants (Fig. 1 B–D). After immunoprecipitation of the HA-tagged Ras effector domain mutants and Western blotting for Raf-1, we detected a high level of Ras-Raf-1 association (coprecipitation) in cells expressing wt V12 Ras (Fig. 1B, compare lanes 1 and 2). However, all of the Ras effector domain mutants displayed significantly reduced Ras-Raf association (Fig. 1B, lanes 3–5), as compared with wt V12 Ras. Both V12S35 Ras and V12G37 Ras displayed some Raf-1 association whereas none was observed with the V12C40 Ras mutant. We next tested for activation of MEK by using a phospho-specific MEK1/2 antibody that only recognizes the activated form of MEK. Serum-starved cells expressing the wt V12 Ras protein displayed significantly elevated MEK phosphorylation when compared with parental control cells (Fig. 1C, compare lanes 1 and 2). V12S35 Ras induced MEK phosphorylation above that of control cells but not to the level observed with wt V12 Ras (Fig. 1C, lane 3) whereas neither V12G37 Ras (Fig. 1C, lane 4) or V12C40 Ras (Fig. 1C, lane 5) showed significant MEK phosphorylation above control levels. Reprobing the blot with an antibody against total MEK1/2 showed similar levels of MEK expression in the different mutant cell lines (data not shown). ERK1/2 activity was measured directly in the cells expressing the different Ras effector domain mutants in an in vitro kinase assay (Fig. 1D). Serum-starved NIH 3T3 (490) cells expressing wt V12 Ras exhibited an ≈2.6-fold increase in ERK1/2 activity toward a myelin basic protein substrate when compared with control cells (Fig. 1D, lanes 1 and 2) whereas the V12S35 Ras lysate showed modest ERK1/2 activity above parental cells (≈1.4-fold increase; compare Fig. 1D, lanes 1 and 3). Cells expressing either of the V12G37 (Fig. 1D, lane 4) or V12C40 (Fig. 1D, lane 5) Ras mutants showed only a very slight increase above control cells. Similar results were obtained by using an antibody specific for the phosphorylated (activated) form of ERK1/2 (data not shown). These results show that, similar to previous reports, the V12S35 Ras mutant retains partial Raf-MEK-ERK1/2 activity whereas V12G37 Ras and V12C40 Ras mutants are virtually inactive in this pathway (refs. 13, 14, and 19; L.V.A., unpublished observations).
Figure 1.
Characterization of the Ras-Raf-MEK-ERK1/2 pathway in NIH 3T3 (490) cells transfected with vector alone (lane 1), wt V12 Ras (lane 2), V12S35 Ras (lane 3), V12G37 Ras (lane 4) or V12C40 Ras (lane 5). (A) Western blot analysis of HA-tagged Ras oncoproteins showing comparable levels of expression in cell pools stably expressing the different Ras effector domain mutants. The positions of the HA-tagged Ras proteins are shown. (B) Ras-Raf-1 association (coprecipitation) in cells expressing the different Ras effector domain mutants. Cell lysates first were immunoprecipitated with an HA antiserum, followed by anti-Raf-1 Western blotting. The positions of p74Raf-1 and Ig heavy chains are indicated. (C) The levels of MEK phosphorylation (activation) in cells expressing the different Ras effector domain mutants were determined by Western blotting using an antibody against only phosphorylated MEK1/2. Reprobing the blot with an antibody that recognizes total MEK1/2 demonstrated the same level of MEK1/2 expression in all samples (data not shown). The positions of phospho-MEK1/2 are shown. (D) ERK1/2 activity in cells expressing the different Ras effector domain mutants. ERK1/2 immunoprecipitates were used in an in vitro kinase assay with myelin basic protein as a substrate in the presence of {γ-32P}-ATP. The position of phosphorylated myelin basic protein is indicated. The positions of the relative molecular mass markers are shown throughout.
Raf-Dependent and Raf-Independent Pathways Contribute to Ras-Mediated Tumorigenesis.
To investigate the tumorigenic properties of cells expressing the different Ras effector domain mutants, we injected stably transfected cells s.c. into athymic nude mice. All Ras effector domain mutant oncogenes were tumorigenic when expressed in NIH 3T3 (490) cells, and only slight differences were observed among the cell lines expressing the various effector domain mutants (Table 1). The V12G37 Ras mutant displayed slightly reduced tumorigenicity when compared with the other mutants, and all effector domain mutants displayed delayed latency periods for tumor growth when compared with wt V12 Ras. The tumors induced by the three Ras effector domain mutants grew in size at comparable rates but slower than those induced by wt V12 Ras (data not shown). These results suggest that Ras-mediated NIH 3T3 tumorigenicity occurs through both Raf-dependent and Raf-independent pathways and that multiple pathways downstream of Ras are required for maximal tumorigenic transformation (14).
Table 1.
Tumorigenic and metastatic properties of NIH 3T3 cells expressing Ras effector domain mutants
| Transfected gene | Tumorigenicity* | Metastasis† |
|---|---|---|
| Vector alone (pDCR) | 1/6 (8)‡ | 0/7 |
| V12 ras (wt) | 6/6 (2) | 9/9 (3–4) |
| V12S35 ras | 6/6 (3) | 7/7 (6–7) |
| V12G37 ras | 7/8 (4)‡ | 0/11 |
| V12C40 ras | 8/8 (3) | 0/11 |
| MEK | 4/4 (3) | 4/4 (5–6) |
| mos | 4/4 (2) | 4/4 (6–8) |
| R-Ras | 3/3 (2–3) | 0/3 |
Number of mice with tumors/total number of mice injected s.c. after 9 weeks. Elapsed times (in weeks) after injection before visible tumors appeared are shown in parentheses.
Number of mice with lung metastases/total number of mice injected i.v. after 14 weeks. Elapsed times (in weeks) after injection before mice displayed signs of metastatic lung disease (labored breathing, loss of weight) are shown in parentheses. The presence or absence of lung metastases was verified by pathology.
Remaining mice developed tumors after 10–14 weeks.
Differential Abilities of Ras Effector Domain Mutants To Mediate Experimental Metastasis.
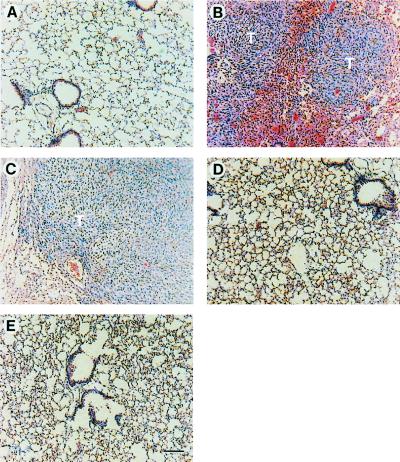
We investigated the ability of cells expressing the various Ras effector domain mutants to colonize the lung in experimental metastasis assays in athymic nude mice. This assay at least measures extravasation and colonization of tumor cells within the lung. In contrast to the similar tumorigenic properties of the Ras effector domain mutants, dramatic differences in the metastatic ability of cells expressing these mutants were observed (Table 1). Cells expressing wt V12 Ras rapidly formed experimental lung metastasis, with the onset of disease occurring at ≈3–4 weeks. Cells expressing the V12S35 Ras mutant formed lung metastases in 100% of mice examined at 6–7 weeks (Table 1). Examination of the lungs from the V12S35 Ras metastases showed that they were of similar grade and morphology when compared with wt V12 Ras (Fig. 2, compare B and C). However, cells expressing either the V12G37 Ras or V12C40 Ras mutants failed to form experimental lung metastases even after 14 weeks. All organs, including the lungs, from these mice were examined and were found to be completely free of micro- or macrometastases (Fig. 2 D and E). These results suggest that activation of the ERK1/2 pathway is key to the development of experimental lung metastases. To test this more directly, we examined the metastatic activities of NIH 3T3 cells transformed with either the Mos oncogene, a potent activator of MEK (20), or a constitutively activated isoform of MEK (16). Both Mos and activated MEK were highly tumorigenic and metastatic in nude mice (Table 1). In addition, NIH 3T3 cells transformed by oncogenic R-Ras (V38 R-Ras), which does not constitutively activate the ERK1/2 pathway in NIH 3T3 cells (ref. 21; data not shown), were found to be highly tumorigenic but not metastatic in experimental assays (Table 1). Taken together, these results suggest that constitutive activation of the ERK1/2 pathway is necessary and sufficient for the acquisition of the metastatic phenotype in NIH 3T3 cells.
Figure 2.
Histological analysis (hematoxylin–eosin staining) of paraffin-embedded lung sections from mice after i.v. injection of cells expressing the different Ras effector domain mutants. Diffuse metastatic fibrosarcoma tumor tissue (T) can be observed in the lungs of mice injected with cells expressing either wt V12 Ras (B) or V12S35 Ras (C) but not in those injected with cells expressing either control vector (A), V12G37 Ras (D), or V12C40 Ras (E), the lungs from which were completely free of metastatic lesions 14 weeks post injection. (Bars = 100 μm.)
Increased ERK1/2 Activation in V12S35 Ras-Mediated Tumors and Metastases.
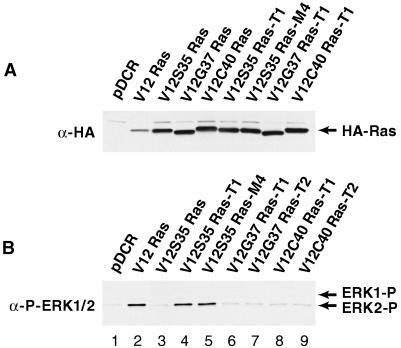
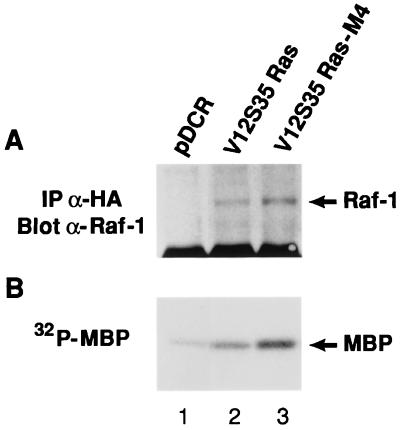
We investigated whether there would be changes in the activation of the Raf-MEK-ERK1/2 pathway in the tumors and metastases derived from NIH 3T3 cells transformed by the different Ras effector domain mutants. Tumor explants derived from s.c. tumors and experimental lung metastases were established as independent cell lines. All tumor- and metastases-derived cell lines were found to express the same level of HA-tagged mutant Ras proteins as the respective parental pools (Fig. 3A). However, a significant increase in ERK1/2 phosphorylation was observed in both the tumor- and metastases-explanted cell lines derived from the V12S35 Ras mutant (Fig. 3B, lanes 3–5). This increase was associated with an increased association between V12S35 Ras and Raf-1 and an increase in ERK1/2 activity (Fig. 4). These results show that V12S35 Ras transformed NIH 3T3 cells expressing high levels of ERK1/2 activity are selected during the formation of tumors and experimental lung metastases. In contrast, ERK1/2 phosphorylation remained low in cell lines derived from the s.c. tumors mediated by the other mutants (V12G37 Ras and V12C40 Ras; Fig. 3B, lanes 6–9). This result further supports the role of ERK1/2-independent signaling pathways during Ras-mediated tumor growth.
Figure 3.
(A) Expression of HA-tagged Ras effector domain mutants in cellular pools and derived s.c. tumors and experimental lung metastases. (B) Activation of ERK1/2 in the tumors and metastases derived from cells transformed by the different Ras effector domain mutants. Western blots were probed with an antibody against the HA-epitope (A) or one that recognizes only activated ERK1/2 (B). Reprobing the blot in B with an antibody against total ERK1/2 demonstrated identical expression in all samples (not shown). The positions of the HA-tagged Ras proteins and phosphorylated ERK1 and ERK2 are indicated. Each cell line is representative of >3 cell lines examined. T, tumor; M, metastases.
Figure 4.
Ras-Raf-1 association (A) and ERK1/2 activity (B) in control cells (lane 1), V12S35 Ras transformed cells (lane 2), and a representative V12S35 Ras-derived metastatic cell line (lane 3). Experiments were performed as described in the legend for Fig. 1 B and D, respectively.
Metastatic Properties of Tumor-Explanted Cell Lines Expressing Ras-Effector Domain Mutants.
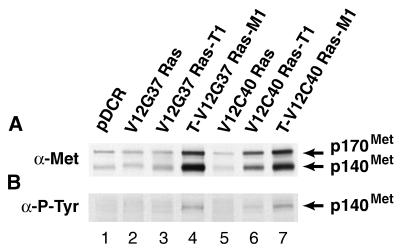
Cell lines derived from explants of the s.c. tumors formed by NIH 3T3 cells expressing the Ras effector domain mutants were reintroduced into nude mice and again were examined for their tumorigenic and metastatic properties. Under these conditions, all mutant tumor cell lines caused rapid s.c. tumor growth comparable to that of wt V12 Ras (Table 2). However, only the V12S35 Ras-explanted tumor cells formed numerous lung metastases comparable to wt V12 Ras (Table 2 and Fig. 5) whereas after 7–9 weeks, the cell lines derived from V12G37 Ras and V12C40 Ras-tumors also produced lung metastases (Table 2). The number of metastatic lesions in the lungs were, however, significantly lower when compared with those observed with V12S35 Ras-tumor-explanted cells (Fig. 5). Tumor explants from the V12G37 and V12C40 metastases (T-V12G37 Ras-M1 and T-V12C40 Ras-M1, respectively) were established and analyzed further. Previous studies (18, 22–24) have shown that expression of the Met receptor tyrosine kinase together with its ligand, hepatocyte growth factor/scatter factor (HGF/SF), results in tumorigenic and metastatic phenotypes in a variety of cell lines and that spontaneous NIH 3T3 metastatic variants overexpress the Met receptor. We, therefore, analyzed the tumor-derived V12G37 Ras and V12C40 Ras metastatic cell lines for expression of the Met receptor. We found a significant increase in Met expression in both metastatic cell lines when compared with both the original cell pools and the tumor cell lines that gave rise to the metastases (Fig. 6). Moreover, Met was reactive with antiphosphotyrosine antibodies in the cell lines derived from the metastases. We suspect that this results from the endogenous expression of HGF/SF in NIH 3T3 cells (22) and that the higher level of Met autophosphorylation may contribute to the metastatic phenotype of these cells.
Table 2.
Tumorigenic and metastatic properties of primary tumor-explanted cells induced by Ras effector domain mutants
| Cell line | Tumorigenicity* | Metastasis† |
|---|---|---|
| T-V12S35 Ras | 4/4 (2) | 4/4 (4–5) |
| T-V12G37 Ras | 4/4 (2–3) | 3/4 (7–9) |
| T-V12C40 Ras | 4/4 (2) | 3/4 (7–8) |
Number of mice with tumors/total number of mice injected s.c. Elapsed times (in weeks) after injection before visible tumors appeared are shown in parentheses.
Number of mice with lung metastases/total number of mice injected i.v. after 14 weeks. Elapsed times (in weeks) after injection before mice displayed signs of metastatic lung disease (labored breathing, loss of weight) are shown in parentheses. The presence or absence of lung metastases was verified by pathology.
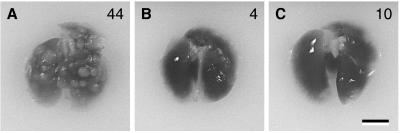
Figure 5.
Photographs of representative lungs (n = 4) of mice injected i.v. with tumor-derived cell lines expressing V12S35 Ras (A), V12G37 Ras (B), and V12C40 Ras (C) after 4 weeks. The values in each panel represent the mean number of metastatic lesions observed at necropsy. (Bar = 0.5 cm.)
Figure 6.
Met expression (A) and Met tyrosine phosphorylation (B) in parental pools (lanes 2 and 5), s.c. tumors (lanes 3 and 6), and tumor-derived metastatic cell lines (lanes 4 and 7) expressing V12G37 Ras (lanes 2–4) and V12C40 Ras (lanes 5–7), along with that in control cells (lane 1). After Met immunoprecipitation, Western blots were probed sequentially for phospho-tyrosine (P-Tyr) and Met. The positions of the p170Met precursor and the p140Met β-chain that contains the tyrosine kinase domain and becomes phosphorylated on activation (33) are shown.
DISCUSSION
A variety of tumor cell–host interactions are involved at multiple levels of the metastatic cascade. The ras oncogene has provided a suitable system for determining the events leading to tumor metastasis by virtue of its ability to induce the metastatic phenotype in a number of experimental systems (1). Much attention has focused recently on the downstream signaling and genetic events leading to the variety of cellular effects mediated by the Ras oncogene. We have provided evidence that the effector pathways mediating the tumorigenic and metastatic activities of Ras can be segregated and that activation of the Raf-MEK-ERK1/2 pathway appears necessary for the acquisition of the metastatic phenotype in NIH 3T3 cells. The effector domain mutant of Ras, which retains partial activation of the Raf-MEK-ERK1/2 pathway (V12S35 Ras), induces NIH 3T3 experimental metastasis whereas those unable to activate this pathway directly (V12G37 Ras and V12C40 Ras) fail to mediate metastasis (Table 1). Although other effectors differentially regulated by these Ras effector domain mutants also may participate in the induction of the metastatic phenotype, we also show that R-Ras-transformed NIH 3T3 cells are tumorigenic but nonmetastatic, coincidental with the inability of oncogenic R-Ras to activate the ERK1/2 pathway (ref. 21; unpublished observations). Furthermore, direct activation of the ERK1/2 pathway, by Mos or a constitutively active mutant of MEK, is sufficient to mediate the metastatic phenotype. In contrast to their different abilities to mediate metastasis, all mutants induce comparable tumorigenic properties when expressed in NIH 3T3 cells (Table 1). In addition, although the tumors derived from V12S35 Ras-transformed cells display elevated activation of ERK1/2, those from V12G37 Ras- and V12C40 Ras-transformed cells showed no increase in active ERK1/2. These data support a previous finding (14) in which it was shown that Ras-mediated tumorigenic transformation occurs through both Raf-dependent and Raf-independent pathways.
Because the abilities of the Ras effector domain mutants to mediate the metastatic phenotype were determined in an experimental assay in which cells are injected directly into the blood stream, it follows that differences in their metastatic propensity must relate to the latter stages of metastasis, i.e., extravasation, establishment, and/or secondary growth within the lung. The precise function of the Raf-MEK-ERK1/2 pathway during these stages of metastasis remains unclear. Oncogenic Ras has been shown to alter the expression profiles of a number of gene products, some of which have been associated with the metastatic phenotype (1). In support of our findings, it was shown recently that activation of ERK1/2 regulates cell motility and migration across extracellular matrix (25) and induces urokinase proteolytic activity (26). The latter enzyme mediates degradation of the extracellular matrix, which is important for the invasive stage of metastasis, and its expression was shown recently to be reduced in squamous cell carcinoma cell lines on treatment with the MEK inhibitor PD 098059, with concomitant reduced invasion in vitro (27). It is interesting to note that it was shown recently that parental and Ras-transformed cells extravasate equally well in a chicken embryo chorioallantoic membrane model (28), suggesting that the effector domain mutants of Ras may differentially regulate postextravasation events, although this remains to be clarified.
Changes in cell shape mediated through modifications of the cell cytoskeleton are likely to play vital roles in the development of the invasive/metastatic phenotype. We routinely observe an increase in the spindle and refractile nature of metastatic cell lines when compared with nonmetastatic cells, and this increase appears to be associated with activation of the ERK1/2 pathway (unpublished observations). It has been shown that certain Ras-induced cytoskeletal changes, particularly membrane ruffling, are mediated through Rac (19, 29), a member of the Rho family of small GTPases, which has been implicated in cell invasion and metastasis (7–10). More recently, the involvement of PI 3-kinase, a known effector of Ras, in Ras-induced membrane ruffling has been demonstrated (30, 31). Of interest, of the three Ras effector domain mutants used in our studies, only V12C40 Ras was shown to activate PI 3-kinase directly and to induce membrane ruffling (19, 31). Thus, our data suggest that direct Ras-mediated activation of PI3-K and subsequent Rac-induced membrane ruffling is not sufficient to induce metastasis. Thus, the V12C40 Ras mutant fails to mediate metastasis, as does R-Ras, which has been shown to efficiently activate PI3-K (21). However, we cannot exclude the possibility that indirect activation of these additional signaling pathways may contribute to the metastatic phenotype.
It is believed widely that metastasis frequently occurs at the late stages of tumor progression. Our results show that the original pools of NIH 3T3 cells transformed by either V12G37 Ras or V12C40 Ras rapidly form s.c. tumors but cannot colonize the lungs in experimental metastasis assays. However, when tumor-derived cell lines are established and reintroduced into nude mice, lung metastases eventually do form after a significant delay (Table 2). These findings suggest that, during the progressive growth of these tumors, a subset of cells acquire metastatic properties. We have shown that these metastatic variants overexpress the Met receptor tyrosine kinase and display Met activation (Fig. 6). We have demonstrated that autocrine Met-HGF/SF activation results in both tumorigenic and metastatic properties in a number of cell systems including NIH 3T3 cells and that spontaneous metastatic variants of NIH 3T3 cells overexpress the Met receptor (18, 22–24). Because Met activates the Ras-ERK1/2 pathway (32) on HGF/SF stimulation, it is possible that the G37/C40 Ras metastatic variants have acquired an indirect means of activating the ERK1/2 pathway through autocrine Met-HGF/SF signaling, thereby promoting metastasis. In this regard, we did observe an increase in Ras-Raf-1 association and a modest elevation in ERK1/2 activation in these metastatic variants (unpublished observations).
Acknowledgments
Thanks to Linda Miller, Marianne Oskarsson, Richard Frederickson, Terry Sweeney, and Oscar Smith for technical assistance. Thanks also to Tracy Lessor, Mike Jeffers, Han-Mo Koo, Shahriar Koochekpour, Nick Duesbery, Michele Fiscella, Monica Murakami, and Greg Taylor for critical review of the manuscript and to Ave Cline for assisting in the preparation of this manuscript. Research sponsored in part by the National Cancer Institute, Department of Health and Human Services, under contract with ABL.
ABBREVIATIONS
- ERK
extracellular regulated kinase
- MAP
mitogen-activated protein
- MEK
MAP kinase kinase
- wt
wild-type
References
- 1.Chambers A F, Tuck A B. Crit Rev Oncog. 1993;4:95–114. [PubMed] [Google Scholar]
- 2.Hunter T. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 3.Hill C S, Treisman R. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 4.Marshall C J. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 5.Vojtek A B, Cooper J A. Cell. 1995;82:527–529. doi: 10.1016/0092-8674(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 6.Van Aelst L, D’Souza-Schorey C. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 7.Michiels F, Habets G G, Stam J C, van der Kammen R A, Collard J G. Nature (London) 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 8.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Nature (London) 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 9.Shaw L M, Rabinovitz I, Wang H H, Toker A, Mercurio A M. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 10.Anand-Apte B, Zetter B R, Viswanathan A, Qiu R-G, Chen J, Ruggieri R, Symons M. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 11.del Peso L, Hernandez-Alcoceba R, Embade N, Carnero A, Esteve P, Paje C, Lacal J C. Oncogene. 1997;15:3047–3057. doi: 10.1038/sj.onc.1201499. [DOI] [PubMed] [Google Scholar]
- 12.Van Aelst L, White M A, Wigler M H. Cold Spring Harbor Symp Quant Biol. 1994;59:181–186. doi: 10.1101/sqb.1994.059.01.022. [DOI] [PubMed] [Google Scholar]
- 13.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 14.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Van Aelst L, Wigler M H, Der C J. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paules R S, Resnick J, Kasenally A B, Ernst M K, Donovan P, Vande Woude G F. Oncogene. 1992;7:2489–2498. [PubMed] [Google Scholar]
- 16.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 17.Webb C P, Lane K, Dawson A P, Vande Woude G F, Warn R M. J Cell Sci. 1996;109:2371–2381. doi: 10.1242/jcs.109.9.2371. [DOI] [PubMed] [Google Scholar]
- 18.Jeffers M, Rong S, Vande Woude G F. Mol Cell Biol. 1996;16:1115–1125. doi: 10.1128/mcb.16.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joneson T, White M A, Wigler M H, Bar-Sagi D. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 20.Posada J, Yew N, Ahn N G, Vande Woude G F, Cooper J A. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marte B M, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Curr Biol. 1996;7:63–70. doi: 10.1016/s0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 22.Rong S, Bodescot M, Blair D, Dunn J, Nakamura T, Mizuno K, Park M, Chan A, Aaronson S, Vande Woude G F. Mol Cell Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong S, Segal S, Anver M, Resau J H, Vande Woude G F. Proc Natl Acad Sci USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffers M, Rong S, Oskarsson M, Anver M, Vande Woude G F. Oncogene. 1996;13:853–861. [PubMed] [Google Scholar]
- 25.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengye E, Singh B, Gum R, Nerlov C, Sabichi A, Birrer M, Boyd D. Oncogene. 1995;11:2639–2648. [PubMed] [Google Scholar]
- 27.Simon C, Juarez J, Nicolson G L, Boyd D. Cancer Res. 1996;56:5369–5374. [PubMed] [Google Scholar]
- 28.Koop S, Schmidt E E, MacDonald I C, Morris V L, Khokha R, Grattan M, Leone J, Chambers A F, Groom A C. Proc Natl Acad Sci USA. 1996;93:11080–11084. doi: 10.1073/pnas.93.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Nature (London) 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 32.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio P M. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 33.Iyer A, Kmiecik T E, Park M, Daar I, Blair D, Dunn K J, Sutrave P, Ihle J N, Bodescot M, Vande Woude G F. Cell Growth Differ. 1990;1:87–95. [PubMed] [Google Scholar]