Abstract
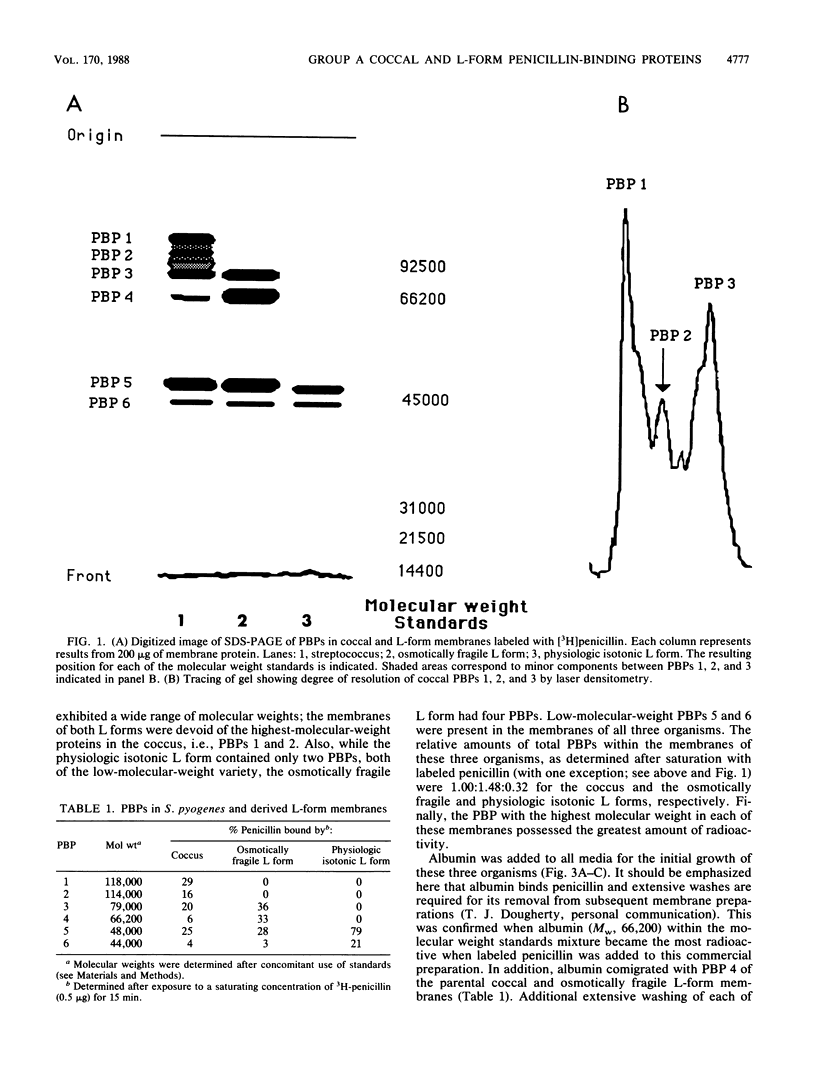
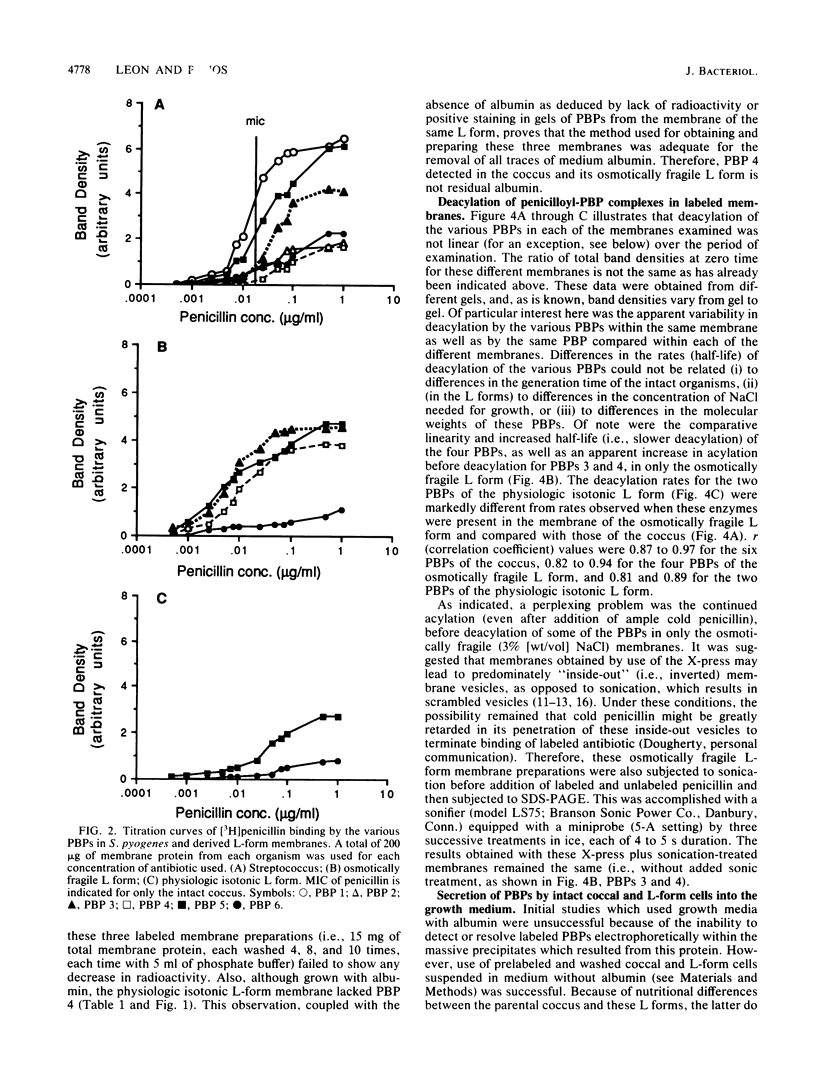
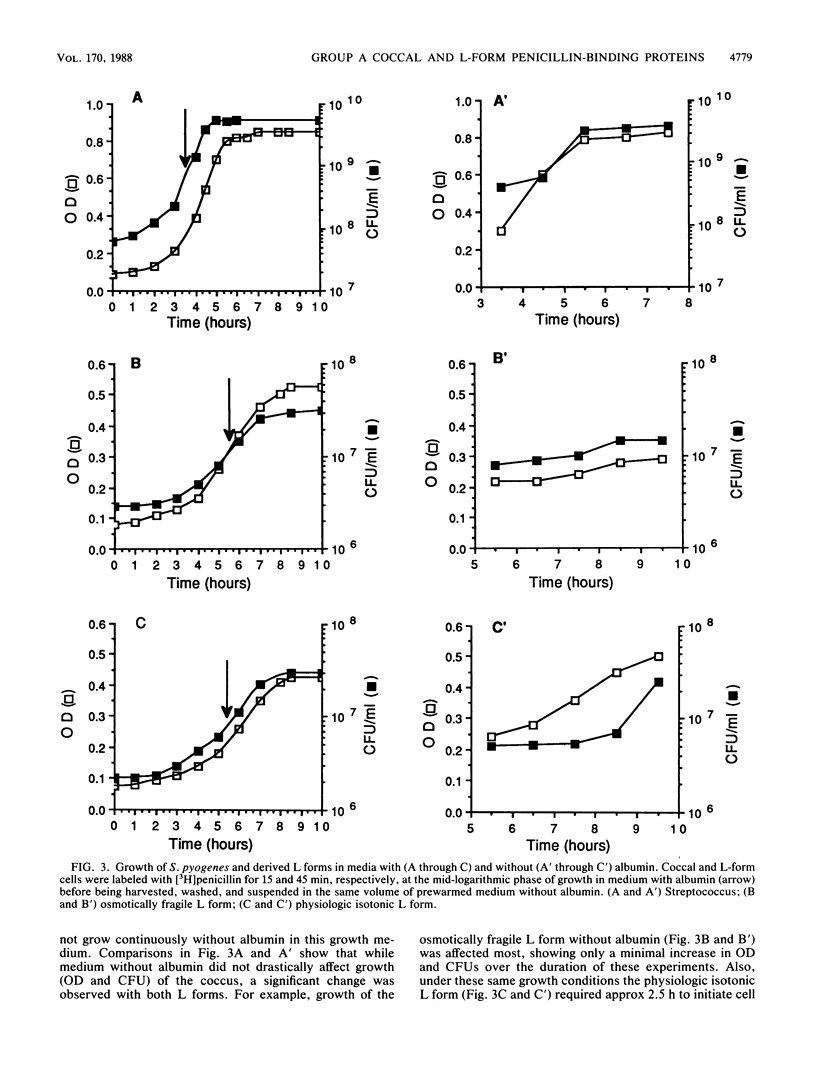
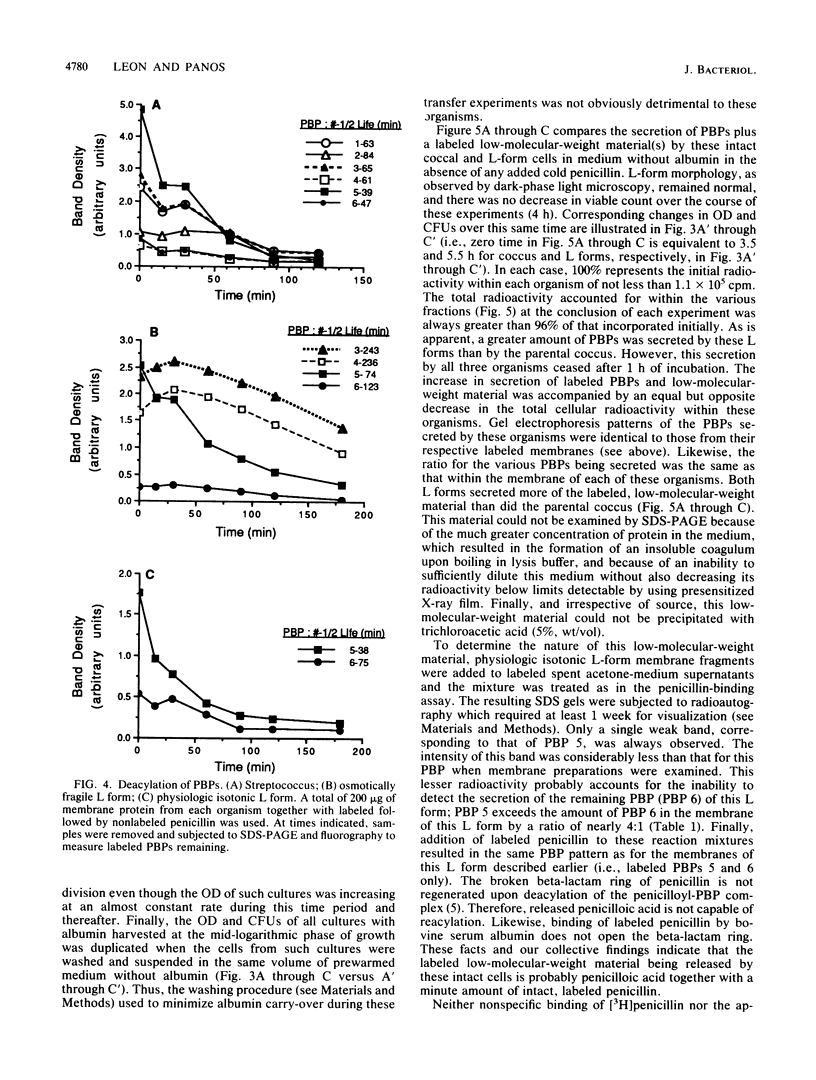
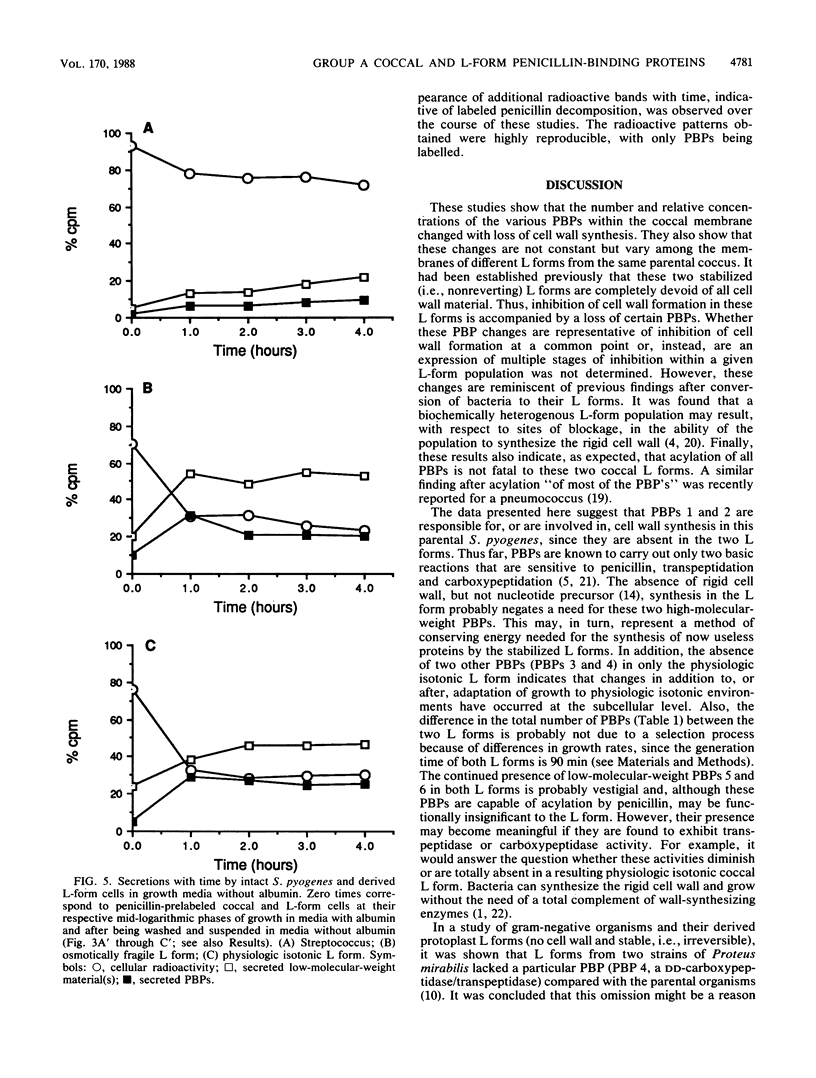
The penicillin-binding proteins (PBPs) of Streptococcus pyogenes and two of its derived, stabilized (i.e., nonreverting) L forms, an osmotically fragile L form and a physiologic isotonic L form, were compared. The numbers of PBPs in the membranes of these organisms were 6, 4, and 2 for the coccus and the osmotically fragile and physiologic isotonic L forms, respectively. Likewise, the relative amounts of total PBPs were 1.00: 1.48:0.32 for this coccus and the osmotically fragile and physiologic isotonic L forms, respectively. The two largest PBPs (PBPs 1 and 2) of the coccus were absent in both L forms, while the smallest PBPs (PBPs 5 and 6) were found in all three membranes. Deacylation (half-life) of three of the four PBPs in the osmotically fragile L form membrane required a significantly longer time than did deacylation of these presumed identical enzymes in the parental coccal membrane. Conversely, there was no such difference between the only two PBPs of the physiologic isotonic L form and the same coccal membrane proteins. Intact cells of all three organisms secreted PBPs and what appeared to be penicilloic acid and a minimal amount of free penicillin. A greater amount of these PBPs was secreted by both L forms than by the coccus. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis patterns and ratios of secreted PBPs were identical to those from labeled membrane preparations. These differences are correlated with some of our previous findings and are discussed in terms of inhibition of cell wall synthesis and resulting membrane changes in these two derived, stabilized coccal L forms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion M., Panos C., Paxton J. Membrane studies of Streptococcus pyogenes and its L-form growing in hypertonic and physiologically isotonic media. An electron spin resonance spectroscopy approach. Biochim Biophys Acta. 1976 Mar 5;426(2):288–301. doi: 10.1016/0005-2736(76)90338-2. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor M. Studies on the cell wall mucopeptide synthesis on staphylococcal L forms. Naturwissenschaften. 1965 Sep;52(18):522–522. doi: 10.1007/BF00638367. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Tomasz A. Physiological properties of penicillin-binding proteins in group A streptococci. Antimicrob Agents Chemother. 1981 May;19(5):872–880. doi: 10.1128/aac.19.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Martin H. H., Schilf W., Schiefer H. G. Differentiation of mycoplasmatales from bacterial protoplast L-forms by assay for penicillin binding proteins. Arch Microbiol. 1980 Oct;127(3):297–299. doi: 10.1007/BF00427207. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Immunochemical analysis of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1413–1422. doi: 10.1021/bi00575a004. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch V. M., Panos C. Defective synthesis of lipid intermediates for peptidoglycan formation in a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1976 Apr;126(1):300–311. doi: 10.1128/jb.126.1.300-311.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., McClees J. S. Active transport of calcium in inverted membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5042–5046. doi: 10.1073/pnas.71.12.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mode of action of beta-lactam antibiotics. Rev Infect Dis. 1979 Jan-Feb;1(1):39–54. doi: 10.1093/clinids/1.1.39. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. Newly made enzymes determine ongoing cell wall synthesis and the antibacterial effects of cell wall synthesis inhibitors. J Bacteriol. 1986 Aug;167(2):535–543. doi: 10.1128/jb.167.2.535-543.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. Peptidoglycan synthesis in L-phase variants of Bacillus licheniformis and Bacillus subtilis. J Bacteriol. 1975 Nov;124(2):668–678. doi: 10.1128/jb.124.2.668-678.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Williamson R., Tomasz A. Inhibition of cell wall synthesis and acylation of the penicillin binding proteins during prolonged exposure of growing Streptococcus pneumoniae to benzylpenicillin. Eur J Biochem. 1985 Sep 16;151(3):475–483. doi: 10.1111/j.1432-1033.1985.tb09126.x. [DOI] [PubMed] [Google Scholar]



