Abstract
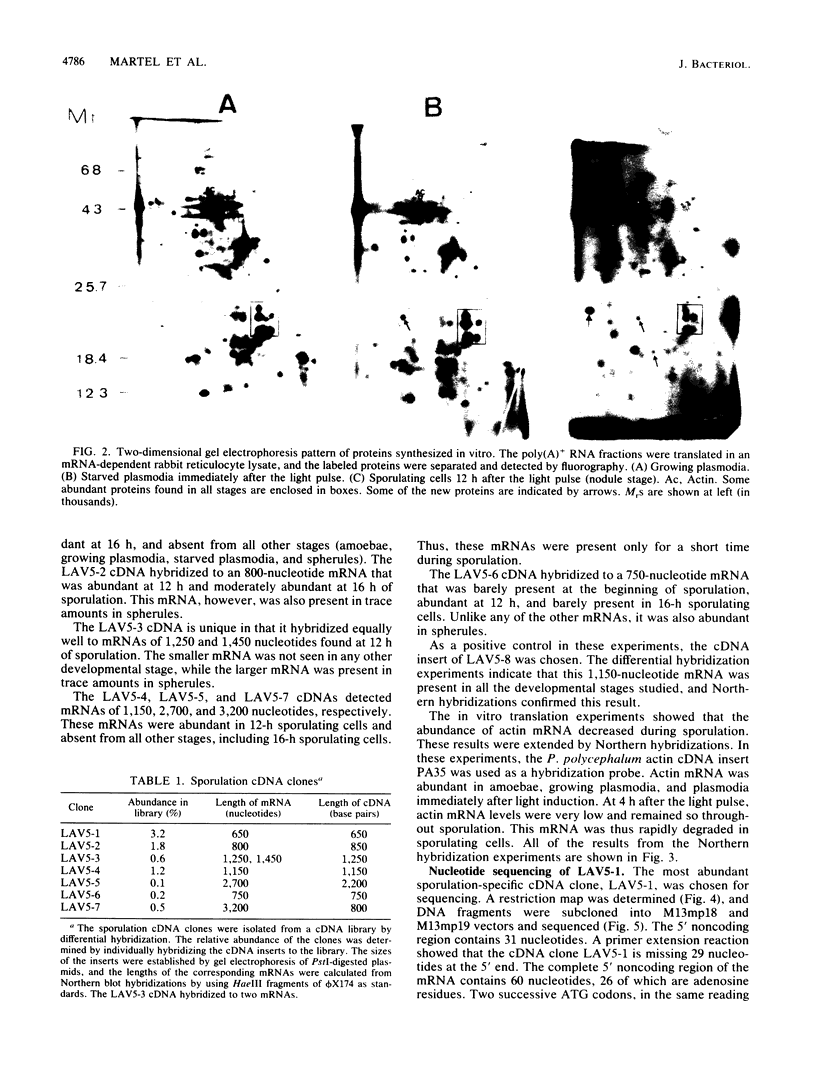
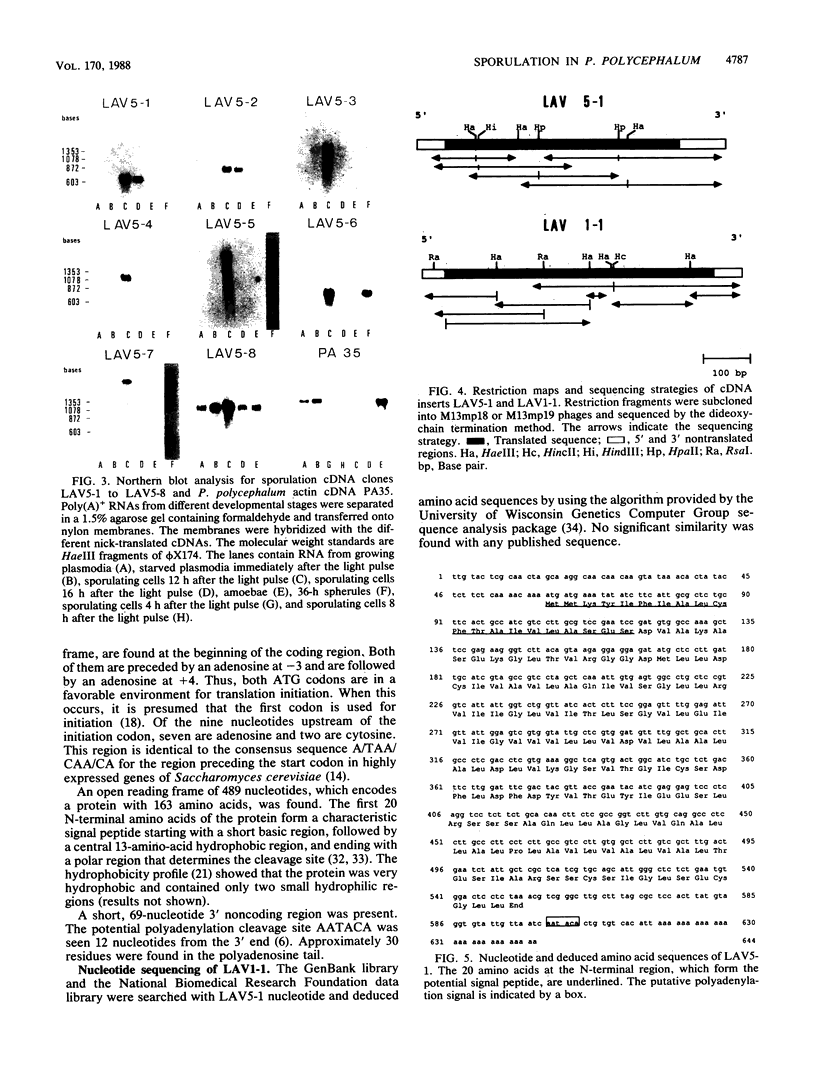
The two-dimensional gel electrophoresis of polypeptides synthesized in vitro from poly(A)+ RNA showed that mRNA populations change during sporulation of Physarum polycephalum. The differential hybridization of a cDNA library prepared from poly(A)+ RNA isolated from sporulating cells revealed that of 846 clones, 64 corresponded to sporulation-specific mRNAs. Further analysis demonstrated that these clones contained seven different sequences: three abundant sequences composing 3.2, 1.8, and 1.2% of the library and four other less abundant sequences. It is probable that all the major mRNAs specifically expressed in early stages of sporulation were identified. The most abundant mRNA from this group coded for a hydrophobic protein that contained a signal peptide. This protein is 47% similar to another Physarum protein, which was encoded by the most abundant plasmodium-specific mRNA. The plasmodial mRNA was degraded during sporulation and was replaced by the sporulation mRNA. These two proteins are thus encoded by members of a gene family whose expression is developmentally regulated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier F., Lemieux G., Pallotta D. Gene families encode the major encystment-specific proteins of Physarum polycephalum plasmodia. Gene. 1987;59(2-3):265–277. doi: 10.1016/0378-1119(87)90334-9. [DOI] [PubMed] [Google Scholar]
- Bernier F., Pallotta D., Lemieux G. Molecular cloning of mRNAs expressed specifically during spherulation of Physarum polycephalum. Biochim Biophys Acta. 1986 Aug 22;867(4):234–243. doi: 10.1016/0167-4781(86)90039-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- DANIEL J. W., RUSCH H. P. Niacin requirement for sporulation of Physarum polycephalum. J Bacteriol. 1962 Jun;83:1244–1250. doi: 10.1128/jb.83.6.1244-1250.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dons J. J., Mulder G. H., Rouwendal G. J., Springer J., Bremer W., Wessels J. G. Sequence analysis of a split gene involved in fruiting from the fungus Schizophyllum commune. EMBO J. 1984 Sep;3(9):2101–2106. doi: 10.1002/j.1460-2075.1984.tb02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTTES E., GUTTES S., RUSCH H. P. Morphological observations on growth and differentation of Physarum polycephalum grown in pure culture. Dev Biol. 1961 Oct;3:588–614. doi: 10.1016/0012-1606(61)90034-3. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Sauer H. W., Brown D. F., Babcock K. L., Rusch H. P. Differential protein synthesis during sporulation in the slime mold Physarum polycephalum. J Bacteriol. 1970 Aug;103(2):356–363. doi: 10.1128/jb.103.2.356-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Lindquist S. Subcellular differentiation in sporulating yeast cells. Cell. 1986 Jun 6;45(5):771–779. doi: 10.1016/0092-8674(86)90791-9. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Miller B. L., Miller K. Y., Roberti K. A., Timberlake W. E. Position-dependent and -independent mechanisms regulate cell-specific expression of the SpoC1 gene cluster of Aspergillus nidulans. Mol Cell Biol. 1987 Jan;7(1):427–434. doi: 10.1128/mcb.7.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Putzer H., Verfuerth C., Claviez M., Schreckenbach T. Photomorphogenesis in Physarum: induction of tubulins and sporulation-specific proteins and of their mRNAs. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7117–7121. doi: 10.1073/pnas.81.22.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Towards a comparative anatomy of N-terminal topogenic protein sequences. J Mol Biol. 1986 May 5;189(1):239–242. doi: 10.1016/0022-2836(86)90394-3. [DOI] [PubMed] [Google Scholar]