Abstract
By insertional mutagenesis, 36 transformation-deficient, mitomycin C-sensitive Bacillus subtilis mutants were isolated, 16 of which were ATP-dependent DNase (ADD) deficient. PBS1 transduction showed that the mutations were closely linked to the metD marker and weakly linked to the glyB marker. With the aid of one of the mutants, a transcription unit involved in ADD synthesis was cloned. The chromosomal location of the transcription unit was established at the restriction site level by determining the presence or absence of ADD in transformants of wild-type cells obtained with various DNA fragments inserted in pUC derivatives. The transcription unit complemented a mutant in which the add transcription unit had been deleted.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amundsen S. K., Taylor A. F., Chaudhury A. M., Smith G. R. recD: the gene for an essential third subunit of exonuclease V. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5558–5562. doi: 10.1073/pnas.83.15.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
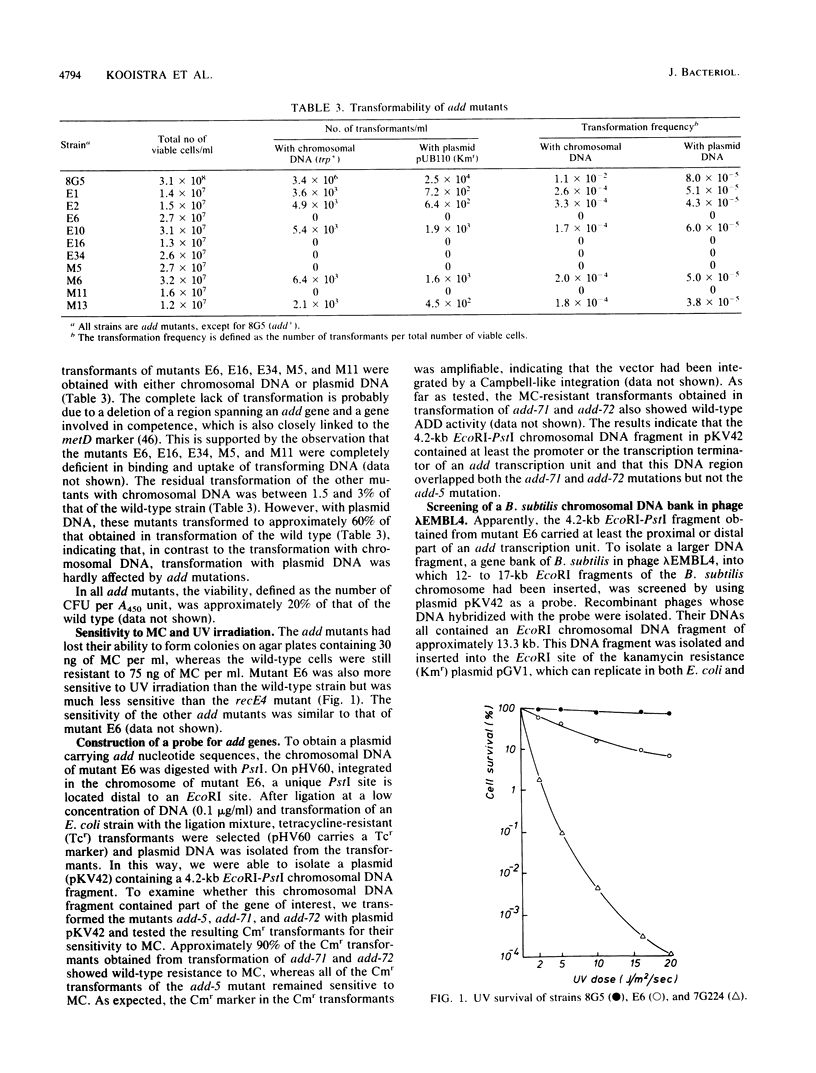
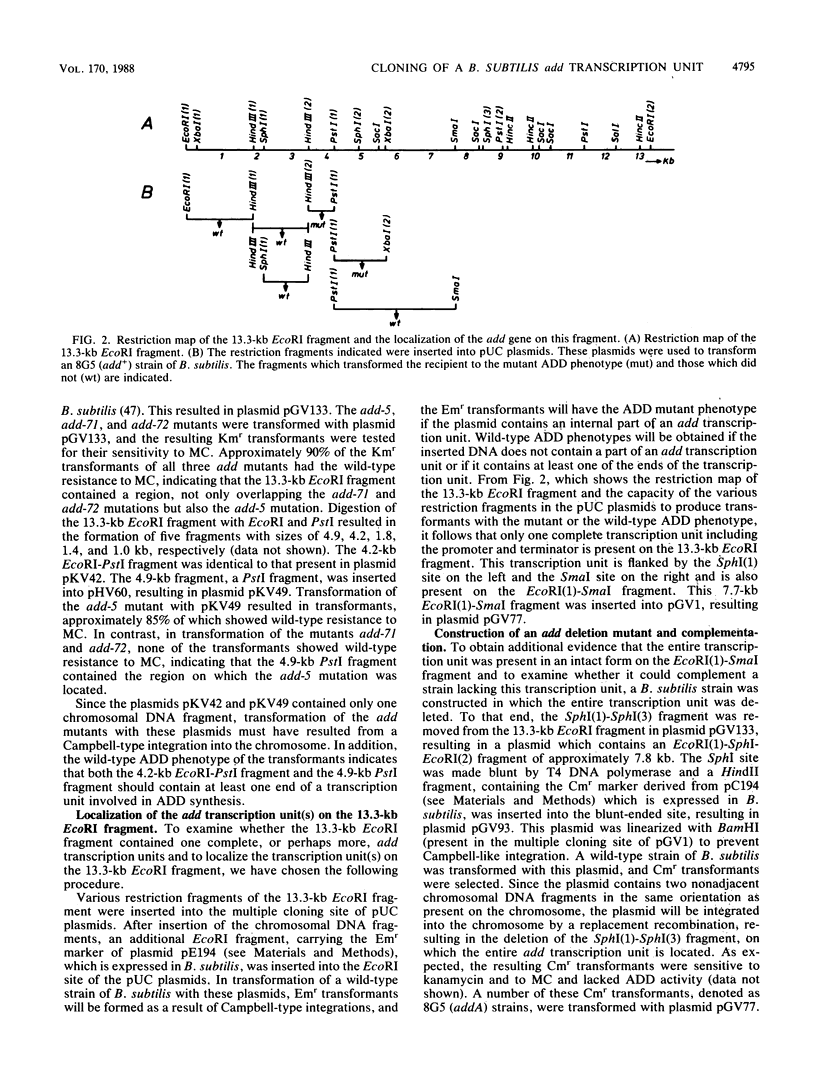
- Bron S., Venema G. Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat Res. 1972 May;15(1):1–10. doi: 10.1016/0027-5107(72)90086-3. [DOI] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A. M., Smith G. R. A new class of Escherichia coli recBC mutants: implications for the role of RecBC enzyme in homologous recombination. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7850–7854. doi: 10.1073/pnas.81.24.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestukhin A. V., Shemyakin M. F., Kalinina N. A., Prozorov A. A. Some properties of ATP dependent deoxyribonucleases from normal and rec-mutant strains of Bacillus subtilis. FEBS Lett. 1972 Jul 15;24(1):121–125. doi: 10.1016/0014-5793(72)80841-x. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly J., Anagnostopoulos C. Isolation, subunit structure and properties of the ATP-dependent deoxyribonuclease of Bacillus subtilis. State of the protein in a mutant devoid of activity. Eur J Biochem. 1976 Dec;71(1):309–316. doi: 10.1111/j.1432-1033.1976.tb11117.x. [DOI] [PubMed] [Google Scholar]
- Doly J., Le Roscouët D., Anagnostopoulos C. Substrate specificity and adenosine triphosphatase activity of the ATP-dependent deoxyribonuclease of Bacillus subtilis. Eur J Biochem. 1981 Mar;114(3):493–499. doi: 10.1111/j.1432-1033.1981.tb05172.x. [DOI] [PubMed] [Google Scholar]
- Doly J., Sasarman E., Anagnostopoulos C. ATP-dependent deoxyribonuclease in Bacillus subtilis and a mutant deficient in this activity. Mutat Res. 1974 Jan;22(1):15–23. doi: 10.1016/0027-5107(74)90003-7. [DOI] [PubMed] [Google Scholar]
- Dykstra C. C., Kushner S. R. Physical characterization of the cloned protease III gene from Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):1055–1059. doi: 10.1128/jb.163.3.1055-1059.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra C. C., Prasher D., Kushner S. R. Physical and biochemical analysis of the cloned recB and recC genes of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):21–27. doi: 10.1128/jb.157.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Wilson R. E., Brown K., Hickson I. D., Emmerson P. T. Complete nucleotide sequence of the Escherichia coli ptr gene encoding protease III. Nucleic Acids Res. 1986 Oct 10;14(19):7695–7703. doi: 10.1093/nar/14.19.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. An endonuclease activity from Escherichia coli absent from certain rec- strains. Proc Natl Acad Sci U S A. 1970 Sep;67(1):434–441. doi: 10.1073/pnas.67.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson I. D., Emmerson P. T. Identification of the Escherichia coli recB and recC gene products. Nature. 1981 Dec 10;294(5841):578–580. doi: 10.1038/294578a0. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Iordănescu S. Recombinant plasmid obtained from two different, compatible staphylococcal plasmids. J Bacteriol. 1975 Nov;124(2):597–601. doi: 10.1128/jb.124.2.597-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S. Relationships between cotransducible plasmids in Staphylococcus aureus. J Bacteriol. 1977 Jan;129(1):71–75. doi: 10.1128/jb.129.1.71-75.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannière L., Niaudet B., Pierre E., Ehrlich S. D. Stable gene amplification in the chromosome of Bacillus subtilis. Gene. 1985;40(1):47–55. doi: 10.1016/0378-1119(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Small G. D., Setlow J. K., Shapanka R. Genetics and complementation of Haemophilus influenzae mutants deficient in adenosine 5'-triphosphate-dependent nuclease. J Bacteriol. 1976 Apr;126(1):31–37. doi: 10.1128/jb.126.1.31-37.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Michel B., Niaudet B., Ehrlich S. D. Intermolecular recombination during transformation of Bacillus subtilis competent cells by monomeric and dimeric plasmids. Plasmid. 1983 Jul;10(1):1–10. doi: 10.1016/0147-619x(83)90052-5. [DOI] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Isolation and partial characterization of Bacillus subtilis mutants impaired in DNA entry. J Bacteriol. 1982 Apr;150(1):260–268. doi: 10.1128/jb.150.1.260-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet B., Goze A., Ehrlich S. D. Insertional mutagenesis in Bacillus subtilis: mechanism and use in gene cloning. Gene. 1982 Oct;19(3):277–284. doi: 10.1016/0378-1119(82)90017-8. [DOI] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosamond J., Telander K. M., Linn S. Modulation of the action of the recBC enzyme of Escherichia coli K-12 by Ca2+. J Biol Chem. 1979 Sep 10;254(17):8646–8652. [PubMed] [Google Scholar]
- Smith H., de Vos W., Bron S. Transformation in Bacillus subtilis: properties of DNA-binding-deficient mutants. J Bacteriol. 1983 Jan;153(1):12–20. doi: 10.1128/jb.153.1.12-20.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Smith G. R. Unwinding and rewinding of DNA by the RecBC enzyme. Cell. 1980 Nov;22(2 Pt 2):447–457. doi: 10.1016/0092-8674(80)90355-4. [DOI] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. FATE OF TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 May;89:1250–1255. doi: 10.1128/jb.89.5.1250-1255.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vosman B., Kooistra J., Olijve J., Venema G. Cloning in Escherichia coli of the gene specifying the DNA-entry nuclease of Bacillus subtilis. Gene. 1987;52(2-3):175–183. doi: 10.1016/0378-1119(87)90044-8. [DOI] [PubMed] [Google Scholar]
- Vosman B., Kooistra J., Olijve J., Venema G. Integration of vector-containing Bacillus subtilis chromosomal DNA by a Campbell-like mechanism. Mol Gen Genet. 1986 Sep;204(3):524–531. doi: 10.1007/BF00331035. [DOI] [PubMed] [Google Scholar]
- de Vos W. M., Venema G. Fate of plasmid DNA in transformation of Bacillus subtilis protoplasts. Mol Gen Genet. 1981;182(1):39–43. doi: 10.1007/BF00422764. [DOI] [PubMed] [Google Scholar]



