Abstract
Vascular endothelial growth factor (VEGF) is a potent and selective vascular endothelial cell mitogen and angiogenic factor. VEGF expression is elevated in a wide variety of solid tumors and is thought to support their growth by enhancing tumor neovascularization. To block VEGF-dependent angiogenesis, tumor cells were transfected with cDNA encoding the native soluble FLT-1 (sFLT-1) truncated VEGF receptor which can function both by sequestering VEGF and, in a dominant negative fashion, by forming inactive heterodimers with membrane-spanning VEGF receptors. Transient transfection of HT-1080 human fibrosarcoma cells with a gene encoding sFLT-1 significantly inhibited their implantation and growth in the lungs of nude mice following i.v. injection and their growth as nodules from cells injected s.c. High sFLT-1 expressing stably transfected HT-1080 clones grew even slower as s.c. tumors. Finally, survival was significantly prolonged in mice injected intracranially with human glioblastoma cells stably transfected with the sflt-1 gene. The ability of sFLT-1 protein to inhibit tumor growth is presumably attributable to its paracrine inhibition of tumor angiogenesis in vivo, since it did not affect tumor cell mitogenesis in vitro. These results not only support VEGF receptors as antiangiogenic targets but also demonstrate that sflt-1 gene therapy might be a feasible approach for inhibiting tumor angiogenesis and growth.
Keywords: angiogenesis/endothelial cells/cancer/gene therapy
Neovascularization is critical for supporting the rapid growth of solid tumors (1). Tumor angiogenesis appears to be achieved by the expression of angiogenic agents within solid tumors that stimulate host vascular endothelial cell mitogenesis and possibly chemotaxis. One such protein, vascular endothelial growth factor (VEGF) or vascular permeability factor (reviewed in refs. 2–4), is a selective endothelial cell mitogen and angiogenic agent that is induced by several growth factors and cytokines, elevated expression of either the ras (5, 6), raf (7), src (8) or mutant P53 (9) oncogenes and hypoxia (10–12) characteristic of rapidly growing solid tumors. VEGF can also be derived from immune cells that infiltrate tumors (13). In addition, expression of the VEGF endothelial cell receptors FLT-1 and KDR/FLK-1 are induced either directly (14) or indirectly (15) by hypoxia. Increased serum VEGF is found in cancer patients (16, 17) and elevated VEGF levels are reported to be a prognostic clinical factor correlated with decreased survival in breast (18), ovarian, (19), lung (20), gastric (21), and colon (22) cancer patients.
Given the potential role of VEGF in promoting tumor angiogenesis, growth, and metastasis, it is an attractive target for therapeutic intervention. This hypothesis is supported by animal experiments in which anti-VEGF monoclonal antibodies are shown to inhibit the growth of implanted tumors (23) and metastatic spread (24–26). Furthermore, either monoclonal antibodies (27) or suppression of VEGF expression (28) results in regression of established tumors. Inhibition of tumor implantation and growth is also achieved by expression of VEGF antisense (29, 30) and of artificially truncated VEGF KDR receptors (31). Although these approaches contribute to the validation of the VEGF system as an attractive therapeutic target and might represent feasible strategies for inhibiting pathological angiogenesis, they all utilize nonnative inhibitors.
The only currently known endogenously expressed selective inhibitor of VEGF is an alternatively spliced version of the FLT-1 VEGF receptor (32). The splicing alteration results in retention of an intron within the mRNA that is translated through to the first in-frame stop codon. This alternatively spliced form of the FLT-1 protein retains the N-terminal six of seven extracellular Ig-like domains fused to the unique intron-encoded 31-amino acid residue C-terminal sequence but is devoid of the membrane proximal Ig-like domain, the membrane spanning polypeptide, and the entire intracellular tyrosine kinase-containing region. The product, denoted soluble FLT-1 (sFLT-1), is a heparin-binding protein that complexes VEGF with the same high affinity and presumably equivalent specificity of the full-length membrane-spanning receptor. It is expressed by vascular endothelial cells and can inhibit their mitogenic response to VEGF in culture by directly sequestering VEGF. In addition, sFLT-1 appears to be able to inhibit the activities of VEGF in a dominant negative manner by heterodimerizing with the extracellular ligand-binding region of the membrane spanning FLT-1 (32) and KDR (33) VEGF receptors, thereby preventing receptor tyrosine transphosphorylation and activation of downstream signal transduction. Therefore, sFLT-1 is a potent and selective endogenous inhibitor of VEGF-mediated angiogenesis. The present set of studies demonstrate that enhanced expression of sFLT-1 by tumor cells in vivo inhibits solid tumor growth, impedes metastatic nodule development, and extends host survival.
MATERIALS AND METHODS
Cell Lines.
The HT-1080 human fibrosarcoma cell line was obtained from the American Type Tissue Culture Collection and the D54-MG human glioblastoma cell line was a gift from Darell Bigner (Duke University, Durham, NC). Cell lines were verified to be Mycoplasma free and maintained in DMEM/Ham’s F-12 plus 10% fetal bovine serum supplemented with 5 mM glutamine and 10 μg/ml ciprofloxacin (complete medium).
Plasmids.
The 2.1-kb sflt-1 cDNA, originally obtained from a human umbilical vein endothelial cell cDNA library (32), was ligated into the BamHI site of the eukaryotic expression vector pcDNA3 (Invitrogen) generating the plasmid psflt-1. The presence and proper orientation of the sflt-1 cDNA were verified by DNA sequencing and restriction analysis. An additional plasmid expressing higher levels of sFLT-1 was constructed by inserting the human cytomegalovirus (HCMV) intron A between the HCMV early promoter and sflt-1 cDNA (pCIsflt-1). A HCMV promoter-driven β-galactosidase cDNA reporter in a pUC 19 plasmid (pLacZ) was used to confirm gene transfer.
Gene Transfections.
For adenovirus–polylysine complex transfection (34, 35), supercoiled plasmid DNA was purified endotoxin-free using the Qiagen megaprep columns (Chatsworth, CA). Adenovirus–polylysine conjugates were prepared by linkage of replication-defective adenovirus to poly-l-lysine as described (35) and diluted to a concentration of 1 × 1011 viral particles/ml. Conjugate DNA complexes were then formed by the sequential addition to 100 μl of adenovirus–polylysine, 6 μg of psflt-1, pcDNA3, or pLacZ plasmid DNA diluted in 200 μl of 20 mM Hepes/150 mM NaCl (pH 7.3), followed by 4 μg of poly-l-lysine diluted in 200 μl of the same Hepes/NaCl solution. Conjugate–DNA complex containing 2 μg of plasmid DNA was incubated for 6 hr at 37°C with HT-1080 cells in 1 ml of medium containing 2% fetal bovine serum, after which 1 ml of complete medium was added to the cells. Greater than 95% transfection efficiency was verified by 5-bromo-4-chloro-3-indolyl-β-d-galactoside staining of pLacZ-transfected cells.
Northern Blot Analysis.
Total RNA from HT-1080 cell lines was isolated by standard procedures with RNAgents total RNA isolation system (Promega) and 10 μg was separated on a 1.2% agarose/formaldehyde gel and transferred to a Hybond-N+ membrane (Amersham). The 634-bp probe, generated from the HindIII–BamHI restriction fragment (bp 1661–2294) of sflt-1, was labeled (36) and hybridized overnight at 42°C with 0.52 × 105 cpm/ml of hybridization solution (6× SSC, 5× Denhardt’s solution, 100 μg/ml sheared, denatured salmon sperm DNA, and 50% formamide; SSC = 0.15 M NaCl, 15 mM sodium citrate). A β-actin probe confirmed comparable RNA sample loads. The membrane was washed three times with 2× SSC/0.1% SDS for 5 min at 23°C followed by three washes with 0.1× SSC/0.1% SDS for 30 min at 50°C, exposed for 5 days, and bands were visualized and quantified on a PhosphorImager (Molecular Dynamics).
Western Blot Analysis.
Conditioned media were generated by culturing 5 × 106 control cells and sflt-1 stably transfected clones each in 10 ml of DMEM/F-12/10% fetal bovine serum for 48 hr, and 1-ml samples were mixed with 50 μl of a 50% slurry of heparin–Sepharose CL-6B (Pharmacia) in PBS and incubated overnight with rocking at 4°C. Beads were harvested by centrifugation, washed three times with PBS, and eluted by boiling in 20 μl of SDS/PAGE sample buffer. Eluted samples were electrophoretically separated on a 10% SDS/PAGE gel, transferred to Immobilon-P (Millipore), and analyzed by Western blotting with an antibody to the N-terminal sequence of sFLT-1 (33).
Covalent Crosslinking.
A 10-μl aliquot of protein eluted from heparin–Sepharose with 40 μl of PBS/1.2 M NaCl was mixed with 10 μl of DMEM/0.2% gelatin and 105 cpm of 125I-labeled VEGF (Amersham), covalently crosslinked with bis(sulfosuccinimidyl) suberate (Pierce), and the products analyzed on SDS/PAGE as described (32).
Cell Proliferation.
To assess the effects of sflt-1 gene transfer on cell proliferation, 105 HT-1080 cells were plated 24 hr after transfection with either psflt-1 or pcDNA3 in three sets of triplicate wells. The cells were counted by trypan blue exclusion 24-, 48-, and 72-hr postplating to measure proliferation rates. A similar experiment was performed for stable clones by plating 104 clonal cells in triplicate wells.
HT-1080 Lung Metastasis.
To assess the effect of transient transfection of psflt-1 or pcDNA3 on HT-1080 lung metastasis, HT-1080 cells were transfected with either psflt-1 or pcDNA3 (2 μg DNA/well), harvested 24 hr later, and counted. Cells were washed and diluted in serum-free medium and injected via tail vein into CB-17 severe combined immunodeficient (SCID) mice. Lungs were harvested 30 days later, fixed in 10% neutral-buffered Formalin, longitudinally trisected, paraffin embedded, and three 5–7-μm-thickness sections were cut at 200-μm intervals from each embedded block. Tissue sections were stained with hematoxylin and eosin, examined for the presence of tumor nodules by a pathologist (G.P.S.) unaware of the treatments, and statistical significance was determined by χ2 analysis.
HT-1080 s.c. Tumor Growth.
HT-1080 cells were transiently transfected with either psflt-1 or pcDNA3 (2 μg DNA/well), harvested after 24 hr, counted, washed in serum-free medium, and injected s.c. into the flanks of athymic nude mice. For clonal experiments, HT-1080 cells were transfected with either pcDNA3 or pCIsflt-1, incubated in complete medium for 2 days, and then supplemented with 800 μg/ml G418 (GIBCO). After at least five passages, cells were subcloned by limiting dilution, expanded, characterized for mitotic rate in vitro, and injected into both flanks of mice to monitor tumor growth. Tumor length and width were used to calculate volume with the formula for a prolate ellipsoid: Volume = (4/3)π(length/2)(width/2)((length + width)/4). Tumor sizes are reported as mean volumes ± SE and statistical differences between groups were determined using Student’s t test.
D54-MG Intracranial Xenografts.
D54-MG human glioma cells were adenovirus–polylysine transfected with either psflt-1 or pcDNA3 and propagated in complete medium containing 400 μg/ml G418 antibiotic for 1 mo to select for stable transfectants. The selected cells were harvested, counted, and resuspended to a final concentration of 107 cells/100 μl in serum-free DMEM/F-12 containing 5% methylcellulose to enhance cell viability. A midline scalp incision was made in CB-17 SCID mice, a 0.5-mm diameter burr hole was drilled 1.5–2.0 mm to the right of the midline and 0.5–1.0 mm posterior to the coronal suture, and cells (5 × 105/5 μl) were stereotactically injected to a depth of 2.5 mm. Mice were monitored twice daily and the statistical significance of differences in survival times among groups was determined using a Kaplan–Meier survival analysis log rank (Mantel–Cox) test.
RESULTS
Human sflt-1 cDNA was cloned into the HCMV-driven mammalian expression vector pcDNA3 generating the plasmid psflt-1. Transcription/translation analysis confirmed the expression of the sflt-1 gene product from the psflt-1 plasmid (not shown). HT-1080 cells were first transiently transfected with either psflt-1 or pcDNA3 using the high efficiency adenovirus–polylysine system (34, 35). Cell proliferation rates of the sflt-1 and control transient transfectants in culture were indistinguishable, with equal division times of 48 hr demonstrating that expression of the sflt-1 gene did not alter replication rates.
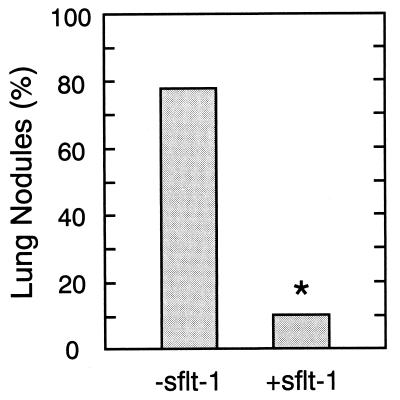
To determine the effect of sFLT-1 expression on tumor cell implantation and growth, immunodeficient nude mice were injected via tail vein with 8 × 105 HT-1080 cells transiently transfected with either psflt-1 or pcDNA3. The lungs of animals sacrificed 30 days later were histologically examined for the presence of tumor nodules. As shown in Fig. 1, seven of nine of the animals injected with pcDNA3-transfected HT-1080 cells developed intrapulmonary tumor nodules identified as clusters of ≥6 cells. In contrast, only one of nine animals injected with HT-1080 cells transiently transfected with psflt-1 developed demonstrable pulmonary nodules (P < 0.02), indicating that even transient expression of sFLT-1 significantly inhibited the implantation and growth of HT-1080 lung metastasis.
Figure 1.
Transient transfection of HT-1080 cells with sflt-1 cDNA inhibits lung implantation and growth. Human HT-1080 fibrosarcoma cells were transiently transfected with plasmid expressing sFLT-1 or the corresponding control plasmid and 8 × 105 cells in 0.3 ml were injected via the tail vein (n = 9/group). After 30 days lungs were removed and examined histologically to detect tumor nodules, defined by clusters containing ≥6 tumor cells. The statistical difference between groups was computed using χ2 statistics (∗, P < 0.02).
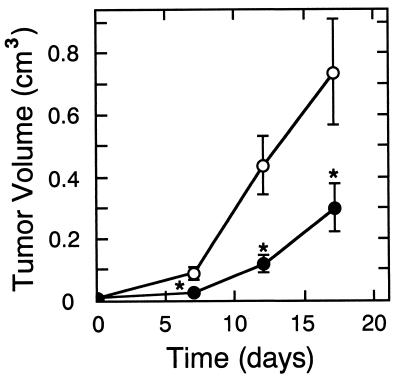
The growth of transiently transfected HT-1080 cells was also examined following s.c. injection into immunodeficient mice. As seen in Fig. 2, s.c. tumors developing from 3 × 106 injected cells expressing sFLT-1 were significantly smaller (P < 0.03) than those formed by an identical number of pcDNA3 control plasmid-transfected tumor cells from days 7 through 17 after implantation. However, psflt-1 tumor nodules grew detectably after the first week, increasing in volume at a rate nearly comparable to the control tumors. Temporary inhibition of growth presumably reflects transient sflt-1 gene expression (35) and, perhaps, transgene dilution on multiple cycles of tumor cell division.
Figure 2.
Transient transfection of HT-1080 cells with sflt-1 cDNA inhibits the growth of s.c. nodules. Human HT-1080 fibrosarcoma cells were transiently transfected with plasmid expressing sFLT-1 (filled circles) or the corresponding control plasmid (open circles). Cells (3 × 106) transfected with either psflt-1 (n = 5) or pcDNA3 (n = 7) were injected into the flanks of athymic nude mice. Nodule dimensions were used to compute tumor volume. The statistical difference between groups was computed using Student’s t test (∗, P < 0.03).
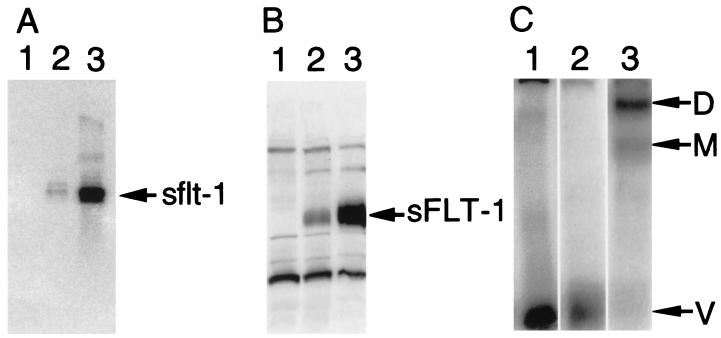
To determine the effect of persistent high sFLT-1 expression on tumor growth, HT-1080 cells were stably transfected with sflt-1 plasmid (pCIsflt-1) incorporating the HCMV intron A, which has been observed to enhance gene expression by ≈10–100-fold (A.J.B., R.L.K., W.R.H., R. C. McFall, and K.A.T., unpublished observations), 3′ of the promoter. These stable transfectants were cloned and individual colonies were characterized for transgene mRNA and protein expression. As shown by the Northern blotting (Fig. 3A), control HT-1080 cells did not express sflt-1 mRNA whereas clones C4 and B3 expressed moderate and 20-fold higher sflt-1 mRNA levels, respectively, as quantified by PhosphorImager analysis. Western blotting of media conditioned by these cells showed a similar pattern of sFLT-1 protein expression (Fig. 3B). As expected, previously observed complexes corresponding to VEGF crosslinked to either one or two molecules of sFLT-1 (32) were detected within conditioned media of the B3 high expresser clone by SDS/PAGE gel analysis. These complexes were not detected in media from either control cells or the lower expressing C4 clone in which they were presumably below the threshold of detection (Fig. 3C).
Figure 3.
Expression of sflt-1 mRNA and sFLT-1 protein by stably transfected HT-1080 tumor cells. In each panel samples derived from control cells stably transfected with pcDNA3 and clones C4 and B3, each stably transfected with pCIsflt-1, are in lanes 1, 2, and 3, respectively. (A) Total cellular RNA was analyzed for the presence of sflt-1 mRNA on a Northern blot hybridized with a specific sflt-1 probe. (B) A Western blot of heparin-binding proteins from media conditioned by these cells was probed with an antisera directed against the N-terminal polypeptide sequence of sFLT-1. (C) Heparin-binding proteins isolated from media conditioned by these cells were covalently crosslinked to 125I-labeled VEGF and analyzed by SDS/PAGE and autoradiography. Arrows indicate the migration positions of free VEGF (V) and VEGF crosslinked to either one (M, monomer) or two (D, dimer) molecules of sFLT-1.
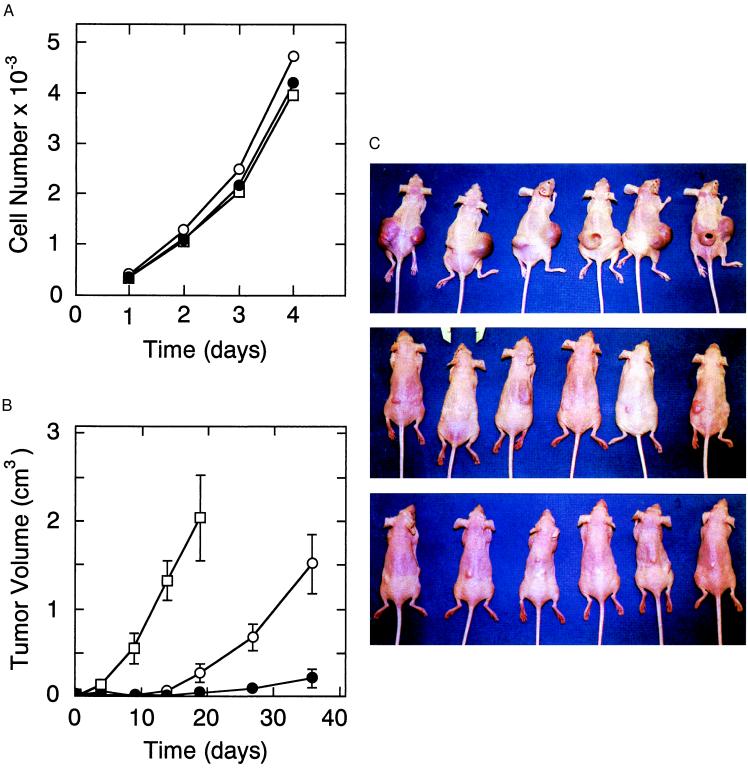
Microscopically, these cell lines were indistinguishable and, as demonstrated in Fig. 4A, had similar growth rates in vitro. These three cell lines were expanded in tissue culture and 3 × 106 cells of each line were injected into the two flanks of six nude mice (12 nodules/clone). As shown in Fig. 4B, the pcDNA3 nodules grew rapidly compared with the clones transfected with the pCIsflt-1 plasmid. On day 19 (Fig. 4C, top), it was necessary to sacrifice the animals injected with pcDNA3 stably transfected HT-1080 control cells because the tumors were large (2.04 ± 0.48 cm3) and beginning to ulcerate. In contrast, clone C4 cells which expressed sFLT-1 grew as significantly (P < 0.001) smaller tumors (Fig. 4C, middle) of 0.278 ± 0.011 cm3 (Fig. 4B). The B3 tumors, expressing greater amounts of sFLT-1, were even smaller (Fig. 4C, bottom) (0.055 ± 0.015 cm3 mean volume) than those arising from the C4 cells (P < 0.05) on day 19 (Fig. 4B). At day 36 (Fig. 4B), the C4 clone mean tumor volume (1.52 ± 0.30 cm3) approached the size of the day 19 pcDNA3 tumors, whereas the B3 tumors remained significantly smaller (0.215 ± 0.106 cm3, P < 0.001). These results demonstrate that higher persistent paracrine expression of sFLT-1 resulted in the slower tumor growth.
Figure 4.
In vitro and in vivo growth of HT-1080 clones stably transfected with sflt-1 cDNA. HT-1080 tumor cells were stably transfected with control pcDNA3 plasmid (open square) or the corresponding pCIsflt-1 plasmid incorporating HCMV intron A (pCIsflt-1) from which clones C4 (open circles) and B3 (filled circles) were selected. (A) In vitro the control (pcDNA3), moderate (C4) sFLT-1-expressing, and high (B3) sFLT-1-expressing clones grew with equivalent rates in culture. (B) In nude mice (n = 6/group) tumors developing from two s.c. injections totaling 3 × 106 cells/animal of either the control (pcDNA3), moderate sFLT-1-expressing (C4), or high (B3) sFLT-1 expressing clones grew at high, intermediate, and low rates, respectively. (C) Tumors are shown in situ 19 days after injections of HT-1080 cells either stably transfected with control plasmid DNA (pcDNA3, top) or clones stably expressing intermediate (C4, middle) and high (B3, bottom) levels of sFLT-1.
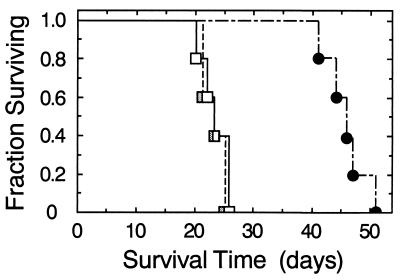
In a second independent tumor model, 5 × 105 human D54-MG glioma cells that were either not transfected or stably transfected with either control or psflt-1 plasmid were stereotactically implanted into the brains of SCID mice to establish the effect of sFLT-1 on mortality rate. D54-MG cells, which are known to produce detectable levels of VEGF (37, 38), grow as brain tumors, leading to death with reproducible kinetics (39). As depicted in Fig. 5, animals injected with either untransfected or pcDNA3-transfected D54-MG cells had median survival times of 24 and 23 days, respectively. In contrast, animals injected with D54-MG expressing sFLT-1 lived almost twice as long with a median survival of 46 days (P < 0.003).
Figure 5.
Survival kinetics of SCID mice injected intracranially with D54-MG human glioma cells stably transfected with sflt-1 cDNA. D54-MG human glioma cells (5 × 105) either not transfected (open squares) or stably transfected with either pcDNA3 (stippled squares) or psflt-1 (filled circles) were stereotactically injected into brains of SCID mice (n = 5/group) to a depth of 2.5 mm after which they were monitored twice daily. Survival of animals injected with tumor cells expressing the sflt-1 gene was significantly (P < 0.005; Kaplan–Meier survival analysis, Mantel–Cox log rank test) longer than those injected with tumor cells transfected with either control pcDNA3 or no plasmid which were not different from each other.
DISCUSSION
Specific fragments of proteins can function as selective inhibitors inasmuch as they are able to antagonistically compete with the functions of their full-length counterparts. In addition to sFLT-1, fragments of several growth factor and cytokine receptors, made by either proteolysis or alternative splicing, and receptor fragment homologues encoded by viruses have been shown to function as specific inhibitors (summarized in ref. 32). Fragments of plasminogen and collagen XVIII, denoted angiostatin (40) and endostatin (41), respectively, also have been demonstrated to inhibit vascular endothelial cell proliferation, angiogenesis, and tumor growth albeit by as yet undefined molecular mechanisms. The molecular targets of sFLT-1 tumor inhibition are the ligands that bind the FLT-1 receptor including VEGF and receptors that can dimerize with sFLT-1 including full-length FLT-1 (32) and KDR (33).
Vascular endothelial cells make both sflt-1 mRNA (32) and the corresponding sFLT-1 protein (33). Recombinant sFLT-1 is a soluble heparin-binding protein that binds VEGF with high affinity (Kd = 20 pM) and a specificity presumably comparable to that of FLT-1. Therefore, sFLT-1 can selectively sequester VEGF much like an antibody but, unlike an antibody, it can also inhibit endothelial cell mitogenesis at concentrations that are substoichiometric to VEGF consistent with a dominant negative inhibition of receptor activation. sFLT-1 can homodimerize, indicating that it can also form dimers with full-length FLT-1 through the equivalent extracellular domains. To date, however, FLT-1 has not been shown to mediate a mitogenic signal in vascular endothelial cells whereas the homologous VEGF receptor KDR is mitogenically competent (reviewed in ref. 3). Recently, we showed that sFLT-1 can heterodimerize with the corresponding extracellular region of KDR (33), thus it should also be able to form heterodimers with full-length KDR.
Growth factor tyrosine kinase receptors appear to function by ligand-mediated dimerization which promotes tyrosine transphosphorylation of the two adjacent intracellular kinase domains generating signal transduction protein docking sites. The efficient inhibition of VEGF by sFLT-1 is compatible with a dominant negative mechanism in which the soluble receptor dimerizes with full-length VEGF receptors preventing receptor tyrosine transphosphorylation, phosphotyrosine-dependent binding of signal transduction proteins, and downstream functional activation. The solubility of sFLT-1 allows it to function in a paracrine manner on vascular endothelial cell receptors while its binding to heparin in vitro is probably indicative of a similar interaction with cell surface heparan proteoglycans which could partition sFLT-1 to the vicinity of plasma membrane-spanning VEGF receptors promoting their dimerization.
Previous studies demonstrate that interference of VEGF-mediated angiogenesis with either anti-VEGF antibodies (24–26) or antisense (29) is sufficient to inhibit metastasis. We now show that in addition to inhibiting VEGF-induced vascular endothelial cell mitogenesis in culture, targeted transient expression of sFLT-1 efficiently inhibits the metastatic implantation and growth of circulating HT-1080 tumor cells. Perhaps sFLT-1 might either inhibit the extravasation of tumor cells by effects on the microvasculature or decrease the survival of implanted cells and the growth of small avascular nodules independent of neovascularization by restricting their access to blood-borne survival factors and growth stimulants through diminished local microvascular permeability.
Both the duration and magnitude of sFLT-1 expression contribute to its inhibition of the growth of primary HT-1080 s.c. tumors. Although transient transfection of HT-1080 cells temporarily inhibits tumor growth in vivo, a substantially longer duration of inhibition is achieved with stable transfection. Furthermore, in an ongoing experiment, tumors grew to the point of ulceration in mice implanted with either control HT-1080 cells or the C4 clone expressing sFLT-1 within 3 wk and 2 mo, respectively, whereas even after 6 mo tumors have not yet grown in half of the animals receiving the B3 clone expressing high levels of sFLT-1.
Intracranial injections of human glioblastoma cells provide a second independent test of the effect of sFLT-1 stable expression. Although brain tumor size could not be readily measured as a function of time, host survival was monitored. Mice receiving cells stably expressing sFLT-1 but not clonally selected for high expression survived approximately twice as long as those receiving either untransfected parental cells or these cells stably transfected with a control plasmid. Therefore, sFLT-1 cannot only inhibit tumor growth and metastatic implantation but also increase host survival.
The mechanism of escape from sFLT-1 inhibition is not yet established. One possibility is that tumor cells are eventually selected that express either diminished sFLT-1 or increased VEGF. These mechanisms are consistent with a longer duration of inhibition of tumor growth by the stably transfected clone expressing high levels of sFLT-1 compared with the parallel clone expressing lower levels. However, in our initial attempts to characterize cells cultured from three independent HT-1080 tumors that eventually arose from implanted sflt-1-stable transfectants, we have not seen a consistent alteration in the expression of either sflt-1 or VEGF mRNA. In cells cultured from one of these tumors we found a 3-fold increase in the sflt-1 mRNA coupled with an 8-fold decrease in sFLT-1 protein, possibly implicating increased proteolytic degradation. Alternatively, tumor cells could arise that express other angiogenic factors that are not inhibited by sFLT-1 but this mechanism would not explain the correlation between the increased level of sFLT-1 expression and the longer duration of tumor growth inhibition.
Although the full spectrum of FLT-1 functions may not yet be known, FLT-1 knockout mice exhibit altered vascular integrity (42). In addition to mediating chemotactic responses of monocytes and perhaps endothelial cells in vitro (43), FLT-1 might modulate endothelial cell mitogenesis either by functioning as a decoy for the mitogenically competent KDR receptor or by forming mitogenically functional receptor heterodimers with it. Unlike a VEGF-specific antibody, the intrinsic ability of sFLT-1 to bind placenta growth factor (44) indicates that it could inhibit the function of not only VEGF/placenta growth factor heterodimers but also placenta growth factor homodimers (45). In principle, sFLT-1 could also be expected to inhibit any other hetero- or homodimeric VEGF homologues that contain FLT-1-binding subunits. Whatever the potential advantages or disadvantages of these other types of inhibition might be, the evolutionary selection of endogenous sFLT-1 expression indicates that it could be a physiologically normal and therapeutically attractive means of inhibiting angiogenesis.
As demonstrated by the sflt-1 transfection results, efficient inhibition of tumor angiogenesis probably requires the continuous presence of an antagonist. One strategy to achieve this goal is to deliver repeatedly either small molecule or macromolecular-specific inhibitors of VEGF receptor activity. Alternatively, sflt-1 gene therapy could be a viable therapeutic approach if continuously expressed in a controlled fashion from stable vectors delivered in vivo. We are currently generating and evaluating the efficacy of persistent viral and cellular vectors stably expressing sFLT-1.
Acknowledgments
We wish to acknowledge Suman Bharara for her technical assistance and Christi Stewart for her secretarial assistance. This work was supported in part by The Daland Fellowship Program at American Philosophical Foundation, The Southern Medical Association, and the Merck Research Laboratories.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: VEGF, vascular endothelial growth factor; sFLT-1, soluble FLT-1; HCMV, human cytomegalovirus.
References
- 1.Folkman J. J Natl Cancer Inst. 1989;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak H F, Brown L F, Detmar M, Dvorak A. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas K. J Biol Chem. 1996;271:603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Davis-Smith T. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 5.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel R S. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 6.Arbisher J L, Moses M A, Fernandez C A, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, et al. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 8.Mukhopadhyay D, Tsiokas L, Sukhatme V P. Cancer Res. 1995;55:6161–6165. [PubMed] [Google Scholar]
- 9.Kieser A, Weich H A, Brandner G, Marme D, Kolch W. Oncogene. 1994;9:963–969. [PubMed] [Google Scholar]
- 10.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 11.Shima D T, Deutsch U, D’Amore P A. FEBS Lett. 1995;370:203–208. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda E, Achen M G, Breier G, Risau W. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- 13.Freeman M R, Schneck F X, Gagnon M L, Corless C, Soker S, Niknejad K, Peoples G E, Klagsbrun M. Cancer Res. 1995;55:4140–4145. [PubMed] [Google Scholar]
- 14.Gerber H-P, Condorelli F, Park J, Ferrara N. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 15.Brogi E, Schatteman G, Wu T, Kim E A, Varticovski L, Keyt B, Isner J. J Clin Invest. 1996;97:469–476. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salven P, Maenpaa H, Orpana A, Alitalo K, Joensuu H. Clin Cancer Res. 1997;3:647–651. [PubMed] [Google Scholar]
- 17.Heits F, Katschinski D M, Wiedemann G J, Weiss C, Jelkmann W. Int J Oncol. 1997;10:333–337. doi: 10.3892/ijo.10.2.333. [DOI] [PubMed] [Google Scholar]
- 18.Toi M, Hoshina S, Takayanagi T, Tominaga T. Jpn J Cancer Res. 1994;85:1045–1049. doi: 10.1111/j.1349-7006.1994.tb02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paley P J, Staskus K A, Gebhard K, Mohanraj D, Twiggs L B, Carson L F, Ramakrishnan S. Cancer (Philadelphia) 1997;80:98–106. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Volm M, Koomagi R, Mattern J. Int J Cancer. 1997;74:64–68. doi: 10.1002/(sici)1097-0215(19970220)74:1<64::aid-ijc11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Maeda K, Chung Y-S, Ogawa Y, Takatsuka S, Kang S-M, Ogawa M, Sawasa T, Sowa M. Cancer (Philadelphia) 1996;77:858–863. doi: 10.1002/(sici)1097-0142(19960301)77:5<858::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, Tucker S L, Kitadai Y, Koura A N, Bucana C D, Cleary K R, Ellis L M. Arch Surg. 1997;132:541–546. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- 23.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 24.Warren R S, Yuan H, Matli M R, Gillett N A, Ferrara N. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melnyk O, Shuman M A, Kim K J. Cancer Res. 1996;56:921–924. [PubMed] [Google Scholar]
- 26.Asano M, Yukita A, Matsumoto T, Kondo S, Suzuki H. Cancer Res. 1995;55:5296–5301. [PubMed] [Google Scholar]
- 27.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain R K. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin L E, Keshet E. Proc Natl Acad Sci USA. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claffey K, Brown L F, del Aquila L F, Tognazzi K, Yeo K-T, Mansuau E J, Dvorak H F. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 30.Cheng S-Y, Huang H-J S, Nagane M, Ji X-D, Wang D, Shih C C-Y, Arap W, Huang C-M, Cavenee W K. Proc Natl Acad Sci USA. 1996;93:8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millauer B, Longhi M P, Plate K H, Shawver L K, Risau W, Ullrich A, Strawn L M. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- 32.Kendall R L, Thomas K A. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall R L, Wang G, Thomas K A. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 34.Curiel D T. Ann NY Acad Sci. 1994;716:36–56. doi: 10.1111/j.1749-6632.1994.tb21702.x. [DOI] [PubMed] [Google Scholar]
- 35.Curiel D T. Prog Med Virol. 1993;40:1–18. [PubMed] [Google Scholar]
- 36.McDaniel T, Meltzer S J. In: Methods in Molecular Biology, PCR Protocols, Current Methods and Applications. White B A, editor. Vol. 15. Totowa, NJ: Humana Press; 1993. pp. 41–43. [Google Scholar]
- 37.Goldman C K, Kim J, Wong W L, King V, Brock T, Gillespie G Y. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai J C, Goldman C K, Gillespie G Y. J Neurosurg. 1995;82:864–873. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- 39.Chambers R, Gillespie G Y, Soroceanu L, Andreansky S, Chatterjee S, Chou J, Roizman B, Whitley R J. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 41.O’Reilly M S, Boehm T, Shing Y, Fukai N, Vasios G, Lane W S, Flynn E, Birkhead J R, Olsen N R, Folkman J. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 42.Fong G-H, Rossant J, Gertsenstein M, Breitman M L. Nature (London) 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 43.Barleon B, Sozzani S, Zhou D, Weich H A, Mantovani A, Marme D. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 44.Kendall R L, Wang G, DiSalvo J, Thomas K A. Biochem Biophys Res Commun. 1994;201:326–330. doi: 10.1006/bbrc.1994.1705. [DOI] [PubMed] [Google Scholar]
- 45.DiSalvo J, Bayne M L, Conn G, Kwok P W, Prashant T G, Soderman D D, Palisi T M, Sullivan K A, Thomas K A. J Biol Chem. 1995;270:7717–7723. doi: 10.1074/jbc.270.13.7717. [DOI] [PubMed] [Google Scholar]