Abstract
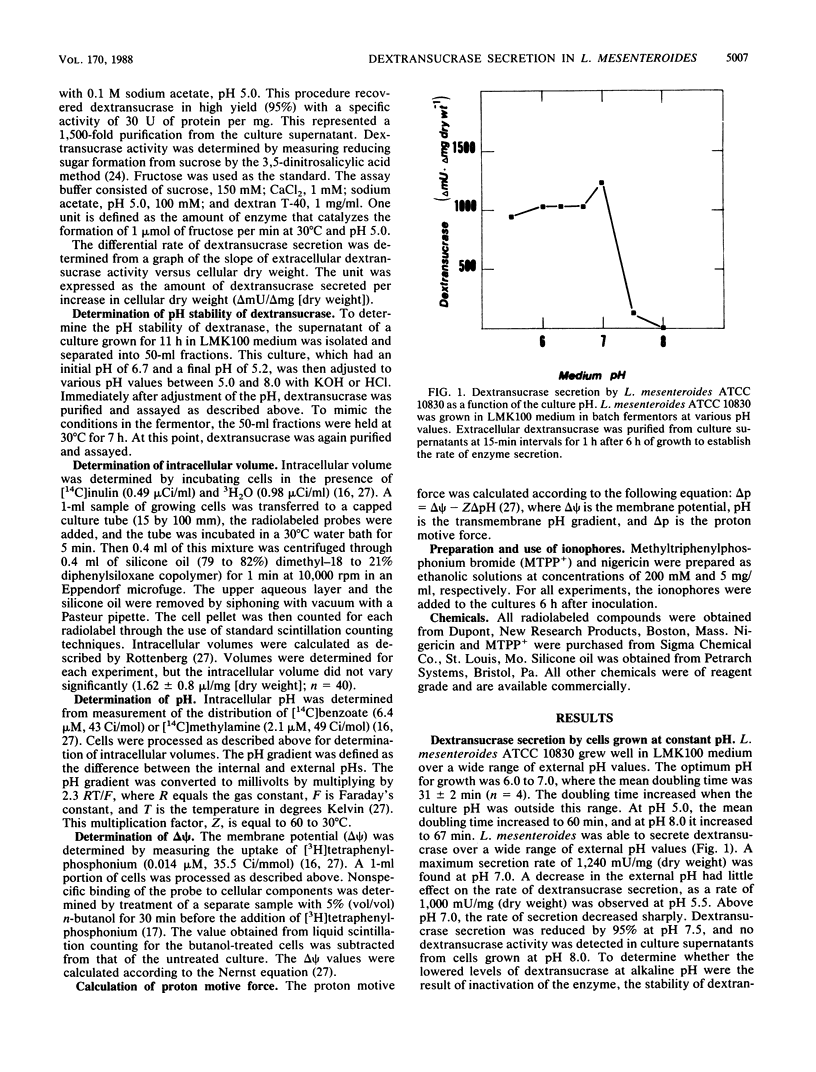
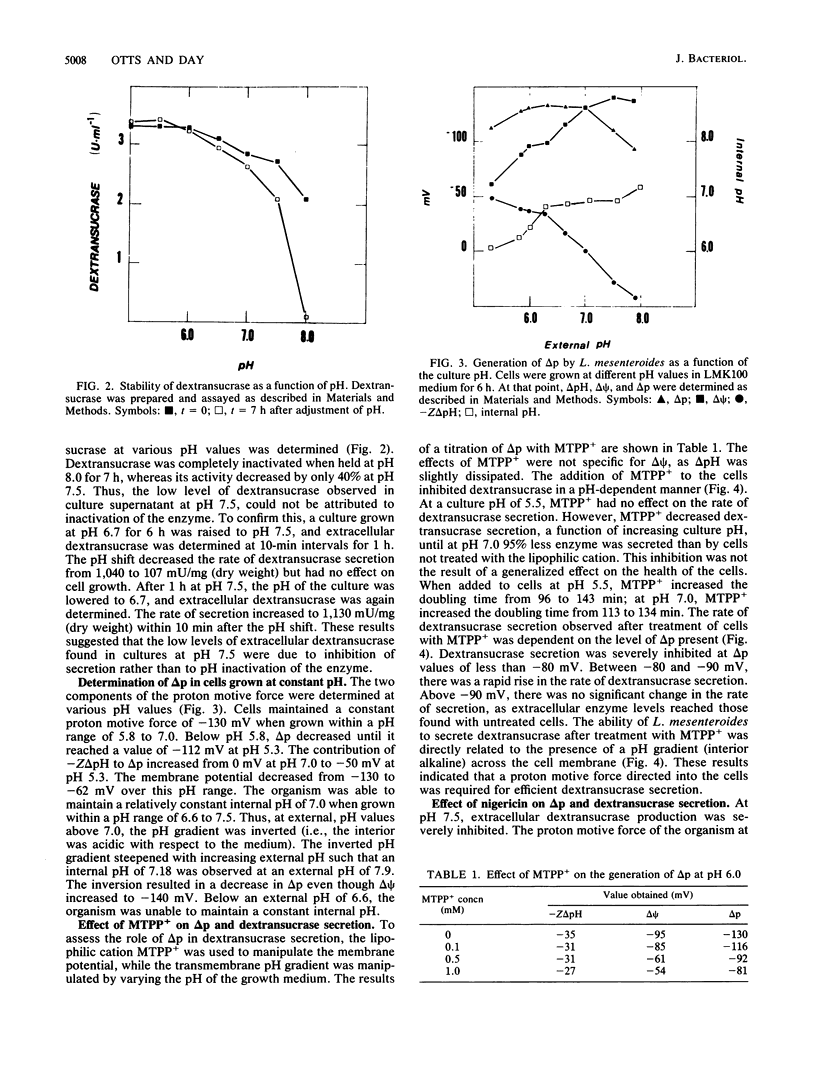
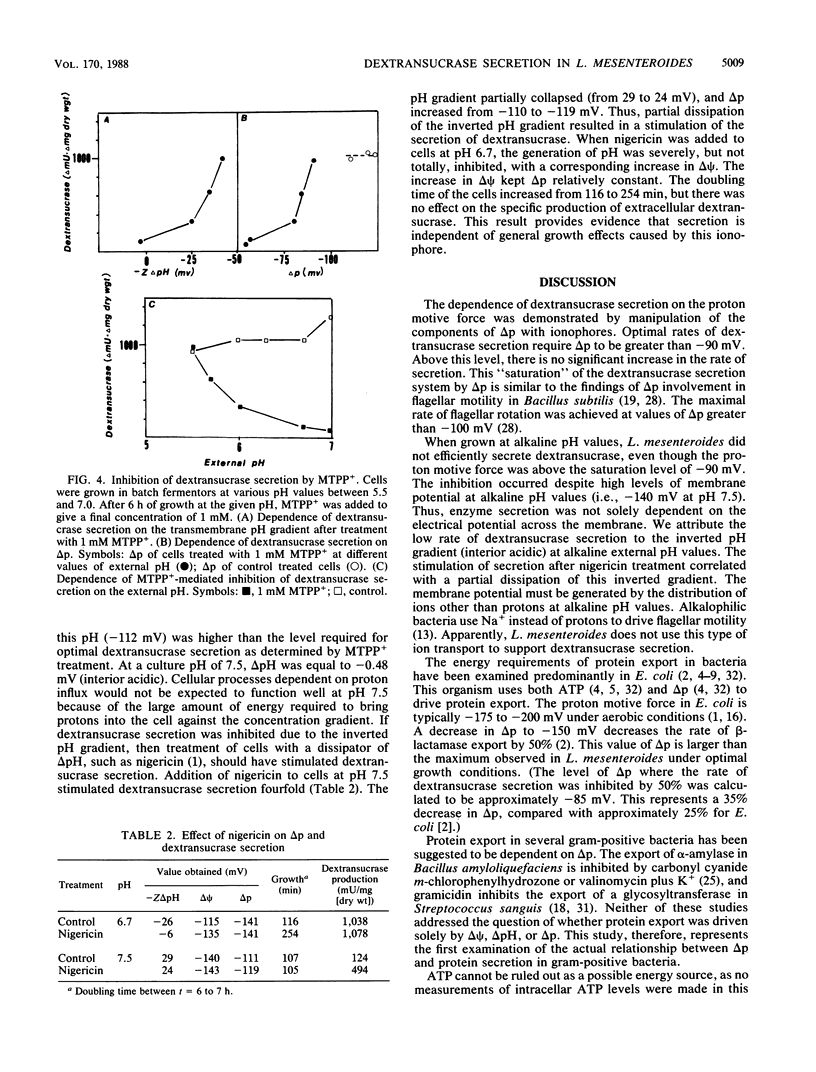
The relationship between proton motive force and the secretion of dextransucrase in Leuconostoc mesenteroides was investigated. L. mesenteroides was able to maintain a constant proton motive force of -130 mV when grown in batch fermentors at pH values 5.8 to 7.0. The contribution of the membrane potential and the transmembrane pH gradient varied depending on the pH of the growth medium. The differential rate of dextransucrase secretion was relatively constant at 1,040 delta mU/delta mg (dry weight) when cells were grown at pH 6.0 to 6.7. Over this pH range, the internal pH was alkaline with respect to the external pH. When cells were grown at alkaline pH values, dextransucrase secretion was severely inhibited. This inhibition was accompanied by an inversion of the pH gradient as the internal pH became more acidic than the external pH. Addition of nigericin to cells at alkaline pH partially dissipated the inverted pH gradient and produced a fourfold stimulation of dextransucrase secretion. Treatment of cells with the lipophilic cation methyltriphenylphosphonium had no effect on the rate of dextransucrase secretion at pH 5.5 but inhibited secretion by 95% at pH 7.0. The reduced rate of secretion correlated with the dissipation of the proton motive force by this compound. Values of proton motive force greater than -90 mV were required for maximal rates of dextransucrase secretion. The results of this study indicate that dextransucrase secretion in L. mesenteroides is dependent on the presence of a proton gradient across the cytoplasmic membrane that is directed into the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Booth I. R. The use of valinomycin, nigericin and trichlorocarbanilide in control of the protonmotive force in Escherichia coli cells. Biochem J. 1983 Apr 15;212(1):105–112. doi: 10.1042/bj2120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. L., Tai P. C. Roles of H+-ATPase and proton motive force in ATP-dependent protein translocation in vitro. J Bacteriol. 1986 Jul;167(1):389–392. doi: 10.1128/jb.167.1.389-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tai P. C. ATP is essential for protein translocation into Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4384–4388. doi: 10.1073/pnas.82.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enequist H. G., Hirst T. R., Harayama S., Hardy S. J., Randall L. L. Energy is required for maturation of exported proteins in Escherichia coli. Eur J Biochem. 1981 May 15;116(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05323.x. [DOI] [PubMed] [Google Scholar]
- Geller B. L., Movva N. R., Wickner W. Both ATP and the electrochemical potential are required for optimal assembly of pro-OmpA into Escherichia coli inner membrane vesicles. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4219–4222. doi: 10.1073/pnas.83.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Van Brunt J. Circulation of H+ and K+ across the plasma membrane is not obligatory for bacterial growth. Science. 1977 Jul 22;197(4301):372–373. doi: 10.1126/science.69317. [DOI] [PubMed] [Google Scholar]
- Harrington C. R., Baddiley J. Synthesis of peptidoglycan and teichoic acid in Bacillus subtilis: role of the electrochemical proton gradient. J Bacteriol. 1984 Sep;159(3):925–933. doi: 10.1128/jb.159.3.925-933.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota N., Imae Y. Na+-driven flagellar motors of an alkalophilic Bacillus strain YN-1. J Biol Chem. 1983 Sep 10;258(17):10577–10581. [PubMed] [Google Scholar]
- Hope M. J., Cullis P. R. Lipid asymmetry induced by transmembrane pH gradients in large unilamellar vesicles. J Biol Chem. 1987 Mar 25;262(9):4360–4366. [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., West A. A., Bourne N., Marsh P. D. Inhibition of the synthesis and secretion of extracellular glucosyl- and fructosyltransferase in Streptococcus sanguis by sodium ions. J Gen Microbiol. 1984 Jan;130(1):77–82. doi: 10.1099/00221287-130-1-77. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Unemoto T., Kobayashi H. Proton motive force is not obligatory for growth of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1074–1077. doi: 10.1128/jb.160.3.1074-1077.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markevics L. J., Jacques N. A. Enhanced secretion of glucosyltransferase by changes in potassium ion concentrations is accompanied by an altered pattern of membrane fatty acids in Streptococcus salivarius. J Bacteriol. 1985 Mar;161(3):989–994. doi: 10.1128/jb.161.3.989-994.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M., Lowe G., Berg H. C. The proton flux through the bacterial flagellar motor. Cell. 1987 Jun 5;49(5):643–650. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Murén E. M., Randall L. L. Export of alpha-amylase by Bacillus amyloliquefaciens requires proton motive force. J Bacteriol. 1985 Nov;164(2):712–716. doi: 10.1128/jb.164.2.712-716.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Ichihara S., Mizushima S. In vitro translocation of protein across Escherichia coli membrane vesicles requires both the proton motive force and ATP. J Biol Chem. 1987 Feb 15;262(5):2358–2362. [PubMed] [Google Scholar]
- ten Brink B., Konings W. N. Electrochemical proton gradient and lactate concentration gradient in Streptococcus cremoris cells grown in batch culture. J Bacteriol. 1982 Nov;152(2):682–686. doi: 10.1128/jb.152.2.682-686.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



