Abstract
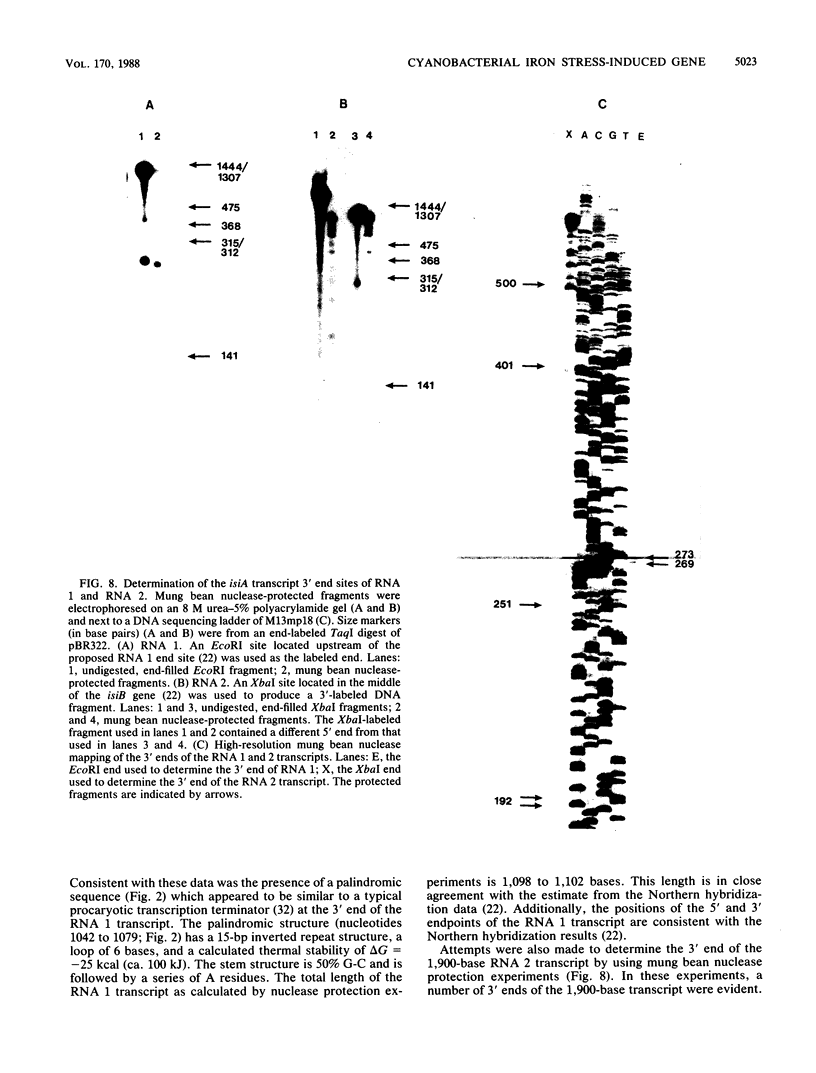
Recently we have reported that the flavodoxin gene from the cyanobacterium Anacystis nidulans R2 is transcribed as part of an iron stress-induced operon containing multiple mRNA species (D. E. Laudenbach, M. E. Reith, and N. A. Straus, J. Bacteriol. 170: 258-265, 1988). Here we report that nucleotide sequence analyses of DNA located immediately upstream of the flavodoxin gene revealed an open reading frame of 1,026 bases (designated isiA; iron stress inducible) with a deduced amino acid sequence showing similarity to that of the psbC polypeptide of higher plants and cyanobacteria. Assuming proteolytic cleavage of the initial methionine residue, the open reading frame encodes a 341-amino-acid polypeptide with a molecular mass of 36,824 daltons. Amino acid sequence comparisons with known psbC polypeptides from spinach and A. nidulans R2 showed extensive similarity, especially in the proposed membrane-spanning regions. Mung bean nuclease mapping and primer extension experiments have localized a transcriptional start site to a position 19 bases upstream from the first methionine codon of the isiA gene product. The upstream region contains an Escherichia coli-like -10 sequence but lacks the typical -35 consensus sequence. Approximately 15, 25, and 150 bases upstream from the isiA transcription start site are 17 base sequences which resemble the operator sequences of iron-regulated genes of E. coli.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Whitaker R. A., Krogmann D. W., Curtis S. E. Isolation and sequence of the gene for ferredoxin I from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1986 Dec;168(3):1265–1271. doi: 10.1128/jb.168.3.1265-1271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Bullerjahn G. S., Matthijs H. C., Mur L. R., Sherman L. A. Chlorophyll-protein composition of the thylakoid membrane from Prochlorothrix hollandica, a prokaryote containing chlorophyll b. Eur J Biochem. 1987 Oct 15;168(2):295–300. doi: 10.1111/j.1432-1033.1987.tb13420.x. [DOI] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. Isolation of PS II reaction centre and its relationship to the minor chlorophyll-protein complexes. J Cell Biochem. 1983;23(1-4):171–179. doi: 10.1002/jcb.240230114. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dandanell G., Hammer K. Two operator sites separated by 599 base pairs are required for deoR repression of the deo operon of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3333–3338. doi: 10.1002/j.1460-2075.1985.tb04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandanell G., Valentin-Hansen P., Larsen J. E., Hammer K. Long-range cooperativity between gene regulatory sequences in a prokaryote. 1987 Feb 26-Mar 4Nature. 325(6107):823–826. doi: 10.1038/325823a0. [DOI] [PubMed] [Google Scholar]
- Dunn T. M., Hahn S., Ogden S., Schleif R. F. An operator at -280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Stearns G. W. Nucleotide sequence and transcript analysis of three photosystem II genes from the cyanobacterium Synechococcus sp. PCC7942. Gene. 1988 Jul 15;67(1):85–96. doi: 10.1016/0378-1119(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Influence of Iron Deprivation on the Membrane Composition of Anacystis nidulans. Plant Physiol. 1984 Jan;74(1):90–95. doi: 10.1104/pp.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Organization and Function of Chlorophyll in Membranes of Cyanobacteria during Iron Starvation. Plant Physiol. 1983 Oct;73(2):250–256. doi: 10.1104/pp.73.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Adams C. W., Belasco J., Doerge B., Cohen S. N. Biological consequences of segmental alterations in mRNA stability: effects of deletion of the intercistronic hairpin loop region of the Rhodobacter capsulatus puf operon. EMBO J. 1987 Nov;6(11):3515–3520. doi: 10.1002/j.1460-2075.1987.tb02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenbach D. E., Reith M. E., Straus N. A. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988 Jan;170(1):258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J., Herrmann R. G. Nucleotide sequence of the gene for the P680 chlorophyll alpha apoprotein of the photosystem II reaction center from spinach. Nucleic Acids Res. 1984 Mar 26;12(6):2837–2850. doi: 10.1093/nar/12.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986 Oct;158(1):165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Riethman H. C., Sherman L. A. Organization of pigment proteins in the photosystem II complex of the cyanobacterium Anacystis nidulans R2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6903–6907. doi: 10.1073/pnas.82.20.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith M. E., Laudenbach D. E., Straus N. A. Isolation and nucleotide sequence analysis of the ferredoxin I gene from the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1986 Dec;168(3):1319–1324. doi: 10.1128/jb.168.3.1319-1324.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S. Light-harvesting II (B800-B850 complex) structural genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1985 Jan;82(1):58–62. doi: 10.1073/pnas.82.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]