Abstract
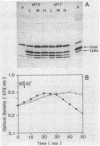
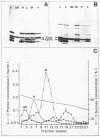
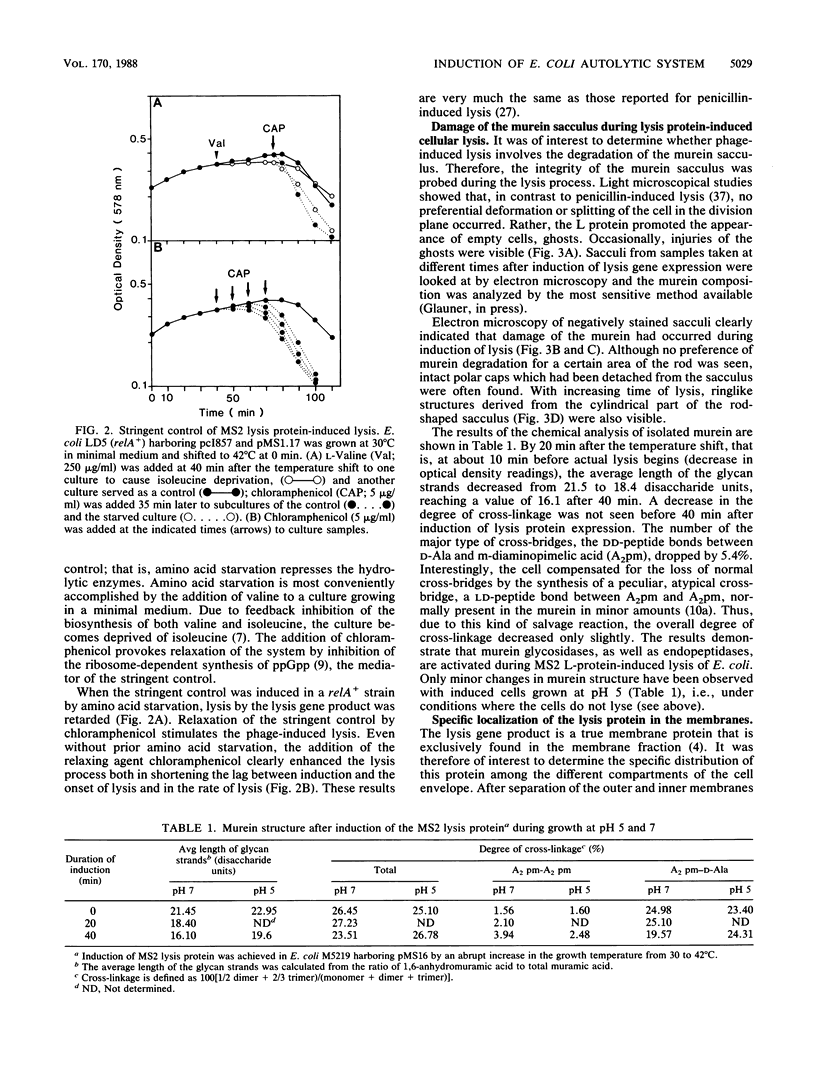
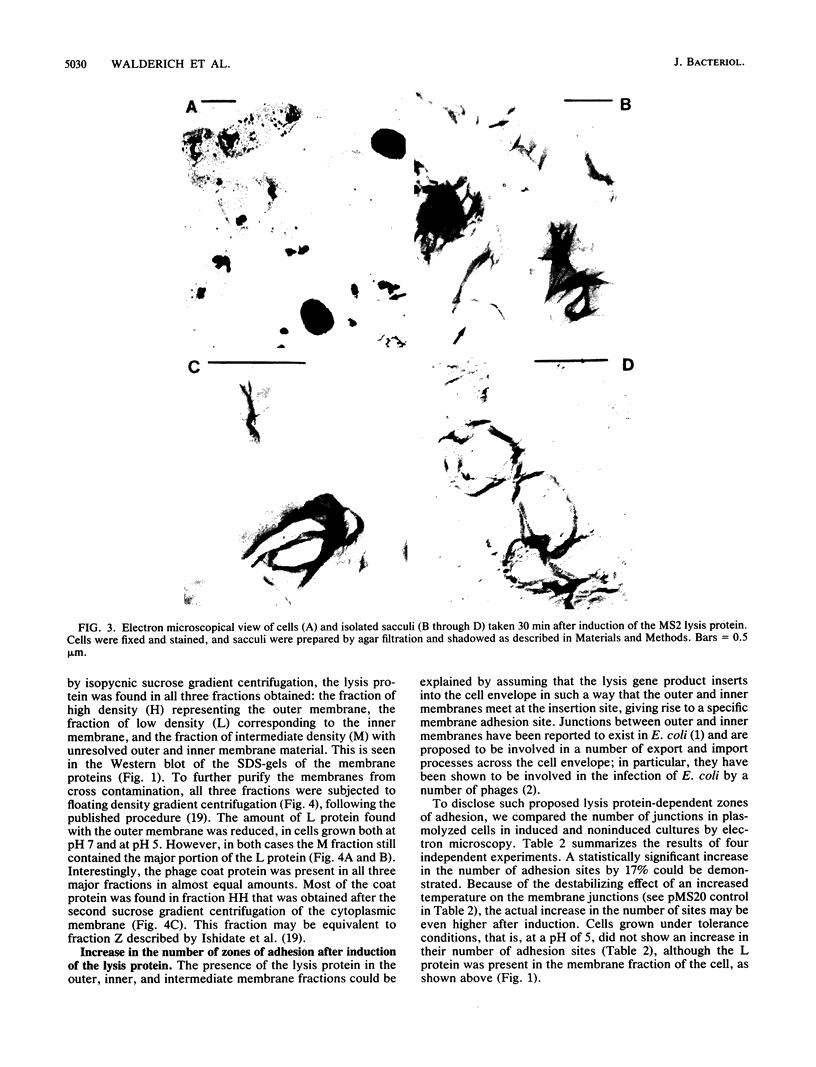
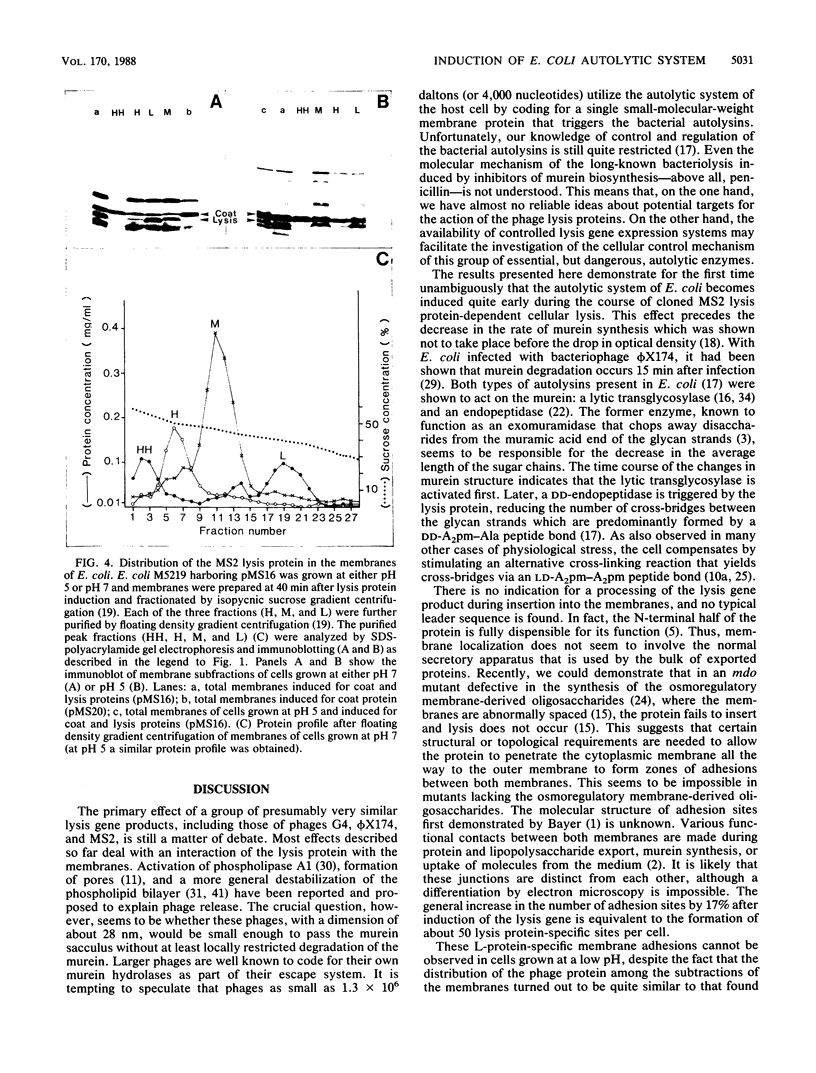
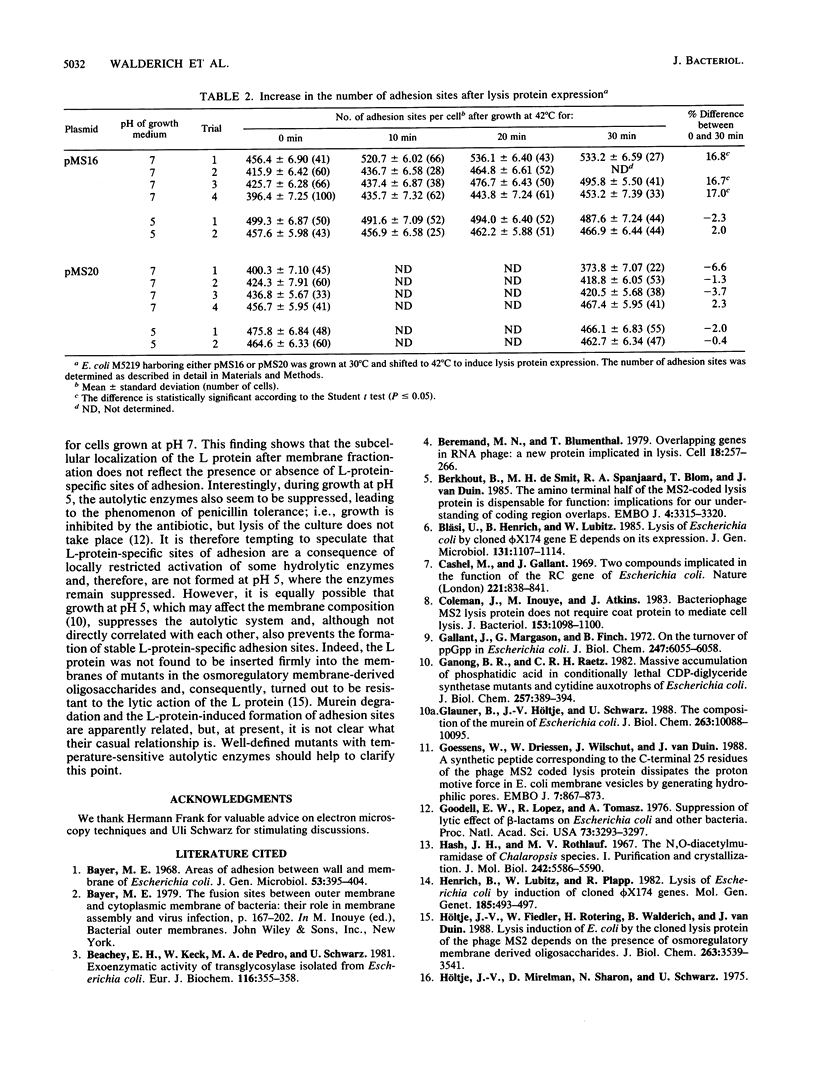
Bacterial lysis induced by the expression of the cloned lysis gene of the RNA bacteriophage MS2 in Escherichia coli was shown to be under the same regulatory control mechanisms as penicillin-induced lysis. It was controlled by the stringent response and showed the phenomenon of tolerance when E. coli was grown at pH 5. Changes in the fine structure of the murein were found to be the earliest physiological changes in the cell, taking place 10 min before the onset of cellular lysis and inhibition of murein synthesis. Both the average length of the glycan strands and, with a time lag, the degree of cross-linkage were altered, indicating that a lytic transglycosylase and a DD-endopeptidase had been triggered. After extensive separation of the membranes by isopycnic sucrose gradient centrifugation, the lysis protein was present predominantly in the cytoplasmic membrane and in a fraction of intermediate density and, to a lesser degree, in the outer membrane, irrespective of the conditions of growth. However, only under lysis-permissive conditions could a 17% increase in the number of adhesion sites between the inner and outer membranes be observed. Thus, a casual relationship between lysis and the formation of lysis protein-induced adhesion sites seems to exist.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Keck W., de Pedro M. A., Schwarz U. Exoenzymatic activity of transglycosylase isolated from Escherichia coli. Eur J Biochem. 1981 May 15;116(2):355–358. doi: 10.1111/j.1432-1033.1981.tb05342.x. [DOI] [PubMed] [Google Scholar]
- Beremand M. N., Blumenthal T. Overlapping genes in RNA phage: a new protein implicated in lysis. Cell. 1979 Oct;18(2):257–266. doi: 10.1016/0092-8674(79)90045-x. [DOI] [PubMed] [Google Scholar]
- Berkhout B., de Smit M. H., Spanjaard R. A., Blom T., van Duin J. The amino terminal half of the MS2-coded lysis protein is dispensable for function: implications for our understanding of coding region overlaps. EMBO J. 1985 Dec 1;4(12):3315–3320. doi: 10.1002/j.1460-2075.1985.tb04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsi U., Henrich B., Lubitz W. Lysis of Escherichia coli by cloned phi X174 gene E depends on its expression. J Gen Microbiol. 1985 May;131(5):1107–1114. doi: 10.1099/00221287-131-5-1107. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Coleman J., Inouye M., Atkins J. Bacteriophage MS2 lysis protein does not require coat protein to mediate cell lysis. J Bacteriol. 1983 Feb;153(2):1098–1100. doi: 10.1128/jb.153.2.1098-1100.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Ganong B. R., Raetz C. R. Massive accumulation of phosphatidic acid in conditionally lethal CDP-diglyceride synthetase mutants and cytidine auxotrophs of Escherichia coli. J Biol Chem. 1982 Jan 10;257(1):389–394. [PubMed] [Google Scholar]
- Glauner B., Höltje J. V., Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10088–10095. [PubMed] [Google Scholar]
- Goessens W. H., Driessen A. J., Wilschut J., van Duin J. A synthetic peptide corresponding to the C-terminal 25 residues of phage MS2 coded lysis protein dissipates the protonmotive force in Escherichia coli membrane vesicles by generating hydrophilic pores. EMBO J. 1988 Mar;7(3):867–873. doi: 10.1002/j.1460-2075.1988.tb02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hash J. H., Rothlauf M. V. The N,O-diacetylmuramidase of Chalaropsis species. I. Purification and crystallization. J Biol Chem. 1967 Dec 10;242(23):5586–5590. [PubMed] [Google Scholar]
- Henrich B., Lubitz W., Plapp R. Lysis of Escherichia coli by induction of cloned phi X174 genes. Mol Gen Genet. 1982;185(3):493–497. doi: 10.1007/BF00334146. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Fiedler W., Rotering H., Walderich B., van Duin J. Lysis induction of Escherichia coli by the cloned lysis protein of the phage MS2 depends on the presence of osmoregulatory membrane-derived oligosaccharides. J Biol Chem. 1988 Mar 15;263(8):3539–3541. [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Stringent control of peptidoglycan biosynthesis in Escherichia coli K-12. J Bacteriol. 1976 Sep;127(3):1119–1126. doi: 10.1128/jb.127.3.1119-1126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- Kastelein R. A., Remaut E., Fiers W., van Duin J. Lysis gene expression of RNA phage MS2 depends on a frameshift during translation of the overlapping coat protein gene. Nature. 1982 Jan 7;295(5844):35–41. doi: 10.1038/295035a0. [DOI] [PubMed] [Google Scholar]
- Keck W., Schwarz U. Escherichia coli murein-DD-endopeptidase insensitive to beta-lactam antibiotics. J Bacteriol. 1979 Sep;139(3):770–774. doi: 10.1128/jb.139.3.770-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W., Glauner B., Höltje J. V. UDP-N-acetylmuramylpentapeptide as acceptor in murein biosynthesis in Escherichia coli membranes and ether-permeabilized cells. J Bacteriol. 1985 Jun;162(3):1000–1004. doi: 10.1128/jb.162.3.1000-1004.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusser W., Ishiguro E. E. Involvement of the relA gene in the autolysis of Escherichia coli induced by inhibitors of peptidoglycan biosynthesis. J Bacteriol. 1985 Nov;164(2):861–865. doi: 10.1128/jb.164.2.861-865.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz W., Harkness R. E., Ishiguro E. E. Requirement for a functional host cell autolytic enzyme system for lysis of Escherichia coli by bacteriophage phi X174. J Bacteriol. 1984 Jul;159(1):385–387. doi: 10.1128/jb.159.1.385-387.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W., Blech J. E. Localization of proteolytic activity in the outer membrane of Escherichia coli. J Bacteriol. 1979 Jan;137(1):574–583. doi: 10.1128/jb.137.1.574-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett H., Keck W., Funk A., Schwarz U. Two different species of murein transglycosylase in Escherichia coli. J Bacteriol. 1980 Oct;144(1):45–52. doi: 10.1128/jb.144.1.45-52.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. D., Young R. Lytic action of cloned phi X174 gene E. J Virol. 1982 Dec;44(3):993–1002. doi: 10.1128/jvi.44.3.993-1002.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]