Abstract
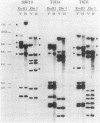
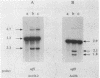
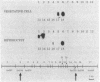
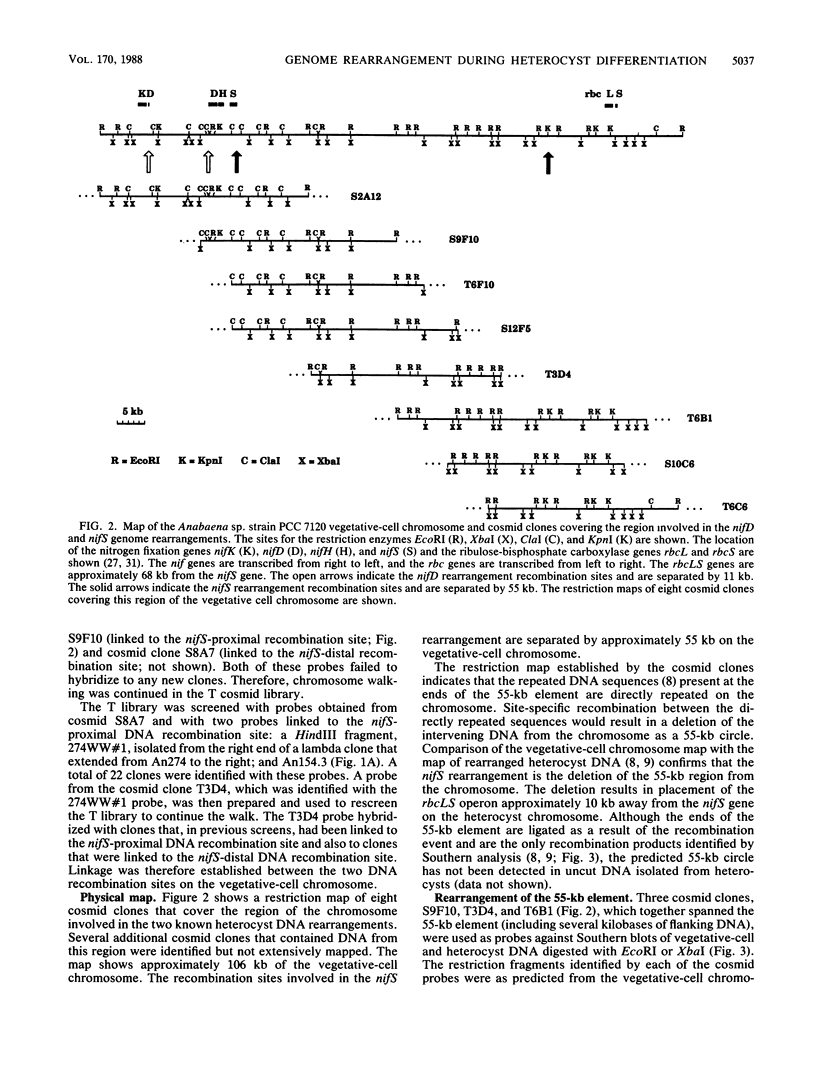
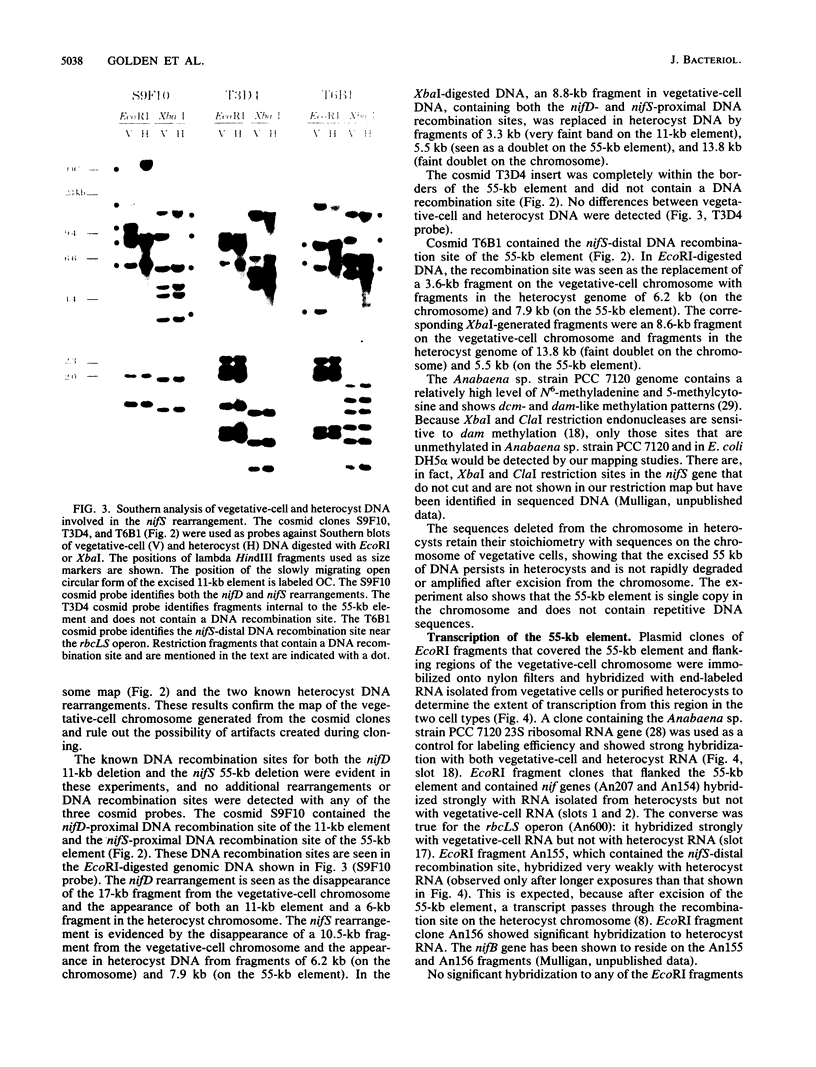
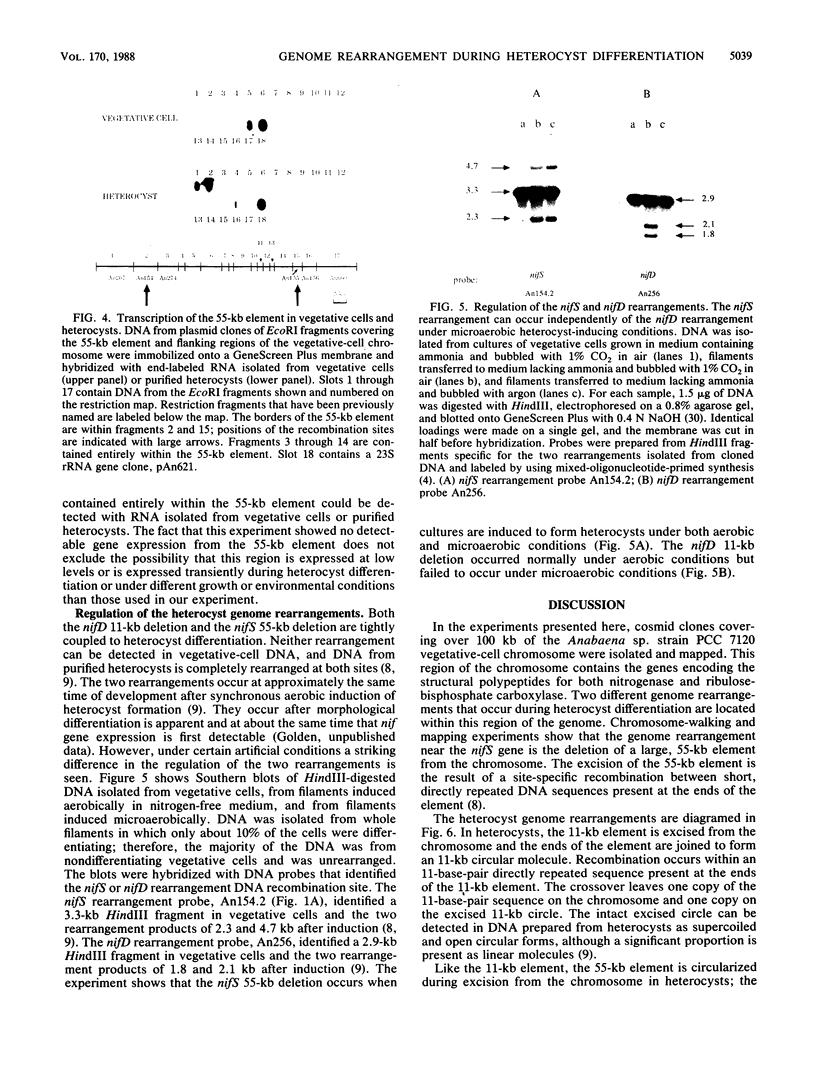
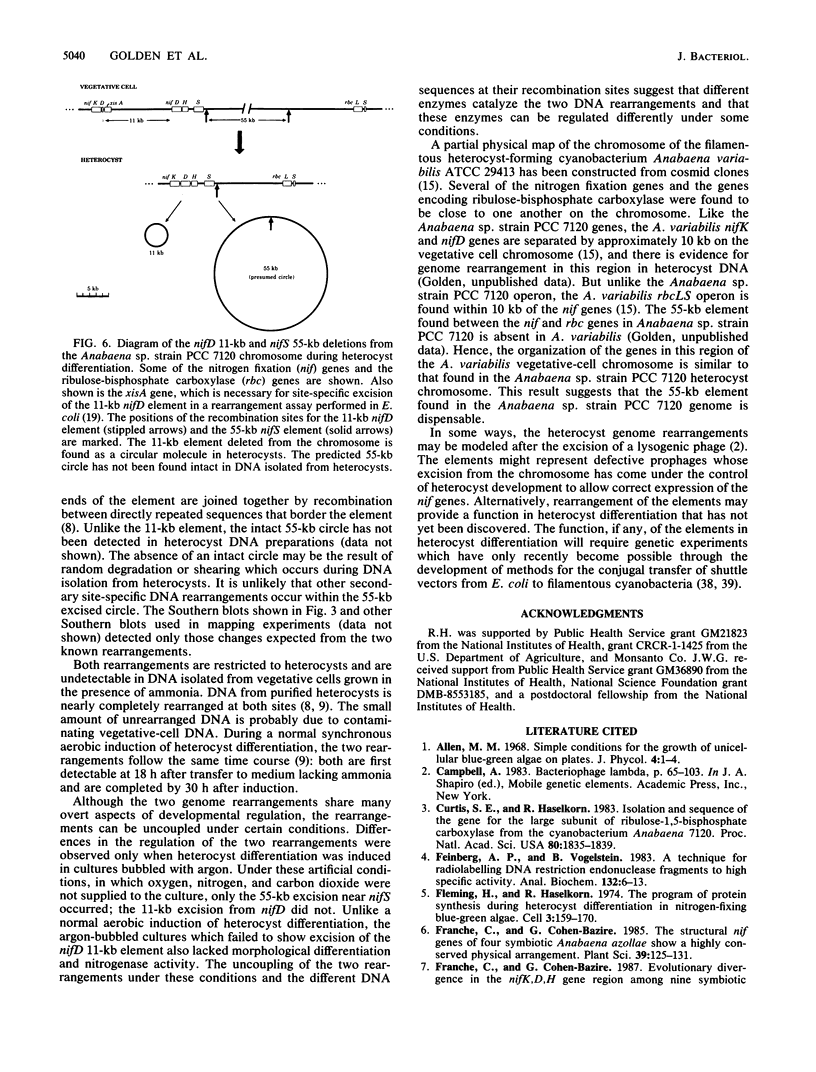
The filamentous cyanobacterium Anabaena sp. strain PCC 7120 produces terminally differentiated heterocysts in response to a lack of combined nitrogen. Heterocysts are found approximately every 10th cell along the filament and are morphologically and biochemically specialized for nitrogen fixation. At least two DNA rearrangements occur during heterocyst differentiation in Anabaena sp. strain PCC 7120, both the result of developmentally regulated site-specific recombination. The first is an 11-kilobase-pair (kb) deletion from within the 3' end of the nifD gene. The second rearrangement occurs near the nifS gene but has not been completely characterized. The DNA sequences found at the recombination sites for each of the two rearrangements show no similarity to each other. To determine the topology of the rearrangement near the nifS gene, cosmid libraries of vegetative-cell genomic DNA were constructed and used to clone the region of the chromosome involved in the rearrangement. Cosmid clones which spanned the DNA separating the two recombination sites that define the ends of the element were obtained. The restriction map of this region of the chromosome showed that the rearrangement was the deletion of a 55-kb DNA element from the heterocyst chromosome. The excised DNA was neither degraded nor amplified, and its function, if any, is unknown. The 55-kb element was not detectably transcribed in either vegetative cells or heterocysts. The deletion resulted in placement of the rbcLS operon about 10 kb from the nifS gene on the chromosome. Although the nifD 11-kb and nifS 55-kb rearrangements both occurred under normal aerobic heterocyst-inducing conditions, only the 55-kb excision occurred in argon-bubbled cultures, indicating that the two DNA rearrangements can be regulated differently.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell. 1974 Oct;3(2):169–170. doi: 10.1016/0092-8674(74)90121-4. [DOI] [PubMed] [Google Scholar]
- Golden G. M., Guzek D. B., Harris R. R., McKie J. E., Potts R. O. Lipid thermotropic transitions in human stratum corneum. J Invest Dermatol. 1986 Mar;86(3):255–259. doi: 10.1111/1523-1747.ep12285373. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Mulligan M. E., Haselkorn R. Different recombination site specificity of two developmentally regulated genome rearrangements. Nature. 1987 Jun 11;327(6122):526–529. doi: 10.1038/327526a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A., Wolk C. P. Genetic mapping of the chromosome of the cyanobacterium, Anabaena variabilis. Proximity of the structural genes for nitrogenase and ribulose-bisphosphate carboxylase. J Biol Chem. 1986 Jun 15;261(17):7748–7754. [PubMed] [Google Scholar]
- Hu N. T., Thiel T., Giddings T. H., Jr, Wolk C. P. New Anabaena and Nostoc cyanophages from sewage settling ponds. Virology. 1981 Oct 15;114(1):236–246. doi: 10.1016/0042-6822(81)90269-5. [DOI] [PubMed] [Google Scholar]
- Kessler C., Neumaier P. S., Wolf W. Recognition sequences of restriction endonucleases and methylases--a review. Gene. 1985;33(1):1–102. doi: 10.1016/0378-1119(85)90119-2. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Golden J. W., Haselkorn R. Identification and sequence of a gene required for a developmentally regulated DNA excision in Anabaena. Cell. 1986 Mar 28;44(6):905–911. doi: 10.1016/0092-8674(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Haselkorn R. Sequence of the nifD gene coding for the alpha subunit of dinitrogenase from the cyanobacterium Anabaena. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4723–4727. doi: 10.1073/pnas.80.15.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Lynn M. E., Bantle J. A., Ownby J. D. Estimation of gene expression in heterocysts of Anabaena variabilis by using DNA-RNA hybridization. J Bacteriol. 1986 Sep;167(3):940–946. doi: 10.1128/jb.167.3.940-946.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Joseph C. M., Haselkorn R. Organization of the nif genes in cyanobacteria in symbiotic association with Azolla and Anthoceros. Arch Microbiol. 1988 May;150(1):61–71. doi: 10.1007/BF00409719. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Haselkorn R. Differences in mRNA levels in Anabaena living freely or in symbiotic association with Azolla. EMBO J. 1986 Jan;5(1):29–35. doi: 10.1002/j.1460-2075.1986.tb04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhy R. N., Hottat F. G., Coene M. M., Hoet P. P. Restriction analysis and quantitative estimation of methylated bases of filamentous and unicellular cyanobacterial DNAs. J Bacteriol. 1988 Apr;170(4):1934–1939. doi: 10.1128/jb.170.4.1934-1939.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Saville B., Straus N., Coleman J. R. Contiguous organization of nitrogenase genes in a heterocystous cyanobacterium. Plant Physiol. 1987 Sep;85(1):26–29. doi: 10.1104/pp.85.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M. Changes in the abundance of polyadenylated RNA during slime mould development measured using cloned molecular hybridization probes. J Mol Biol. 1979 Mar 25;129(1):19–35. doi: 10.1016/0022-2836(79)90056-1. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Cai Y., Cardemil L., Flores E., Hohn B., Murry M., Schmetterer G., Schrautemeier B., Wilson R. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J Bacteriol. 1988 Mar;170(3):1239–1244. doi: 10.1128/jb.170.3.1239-1244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]