Abstract
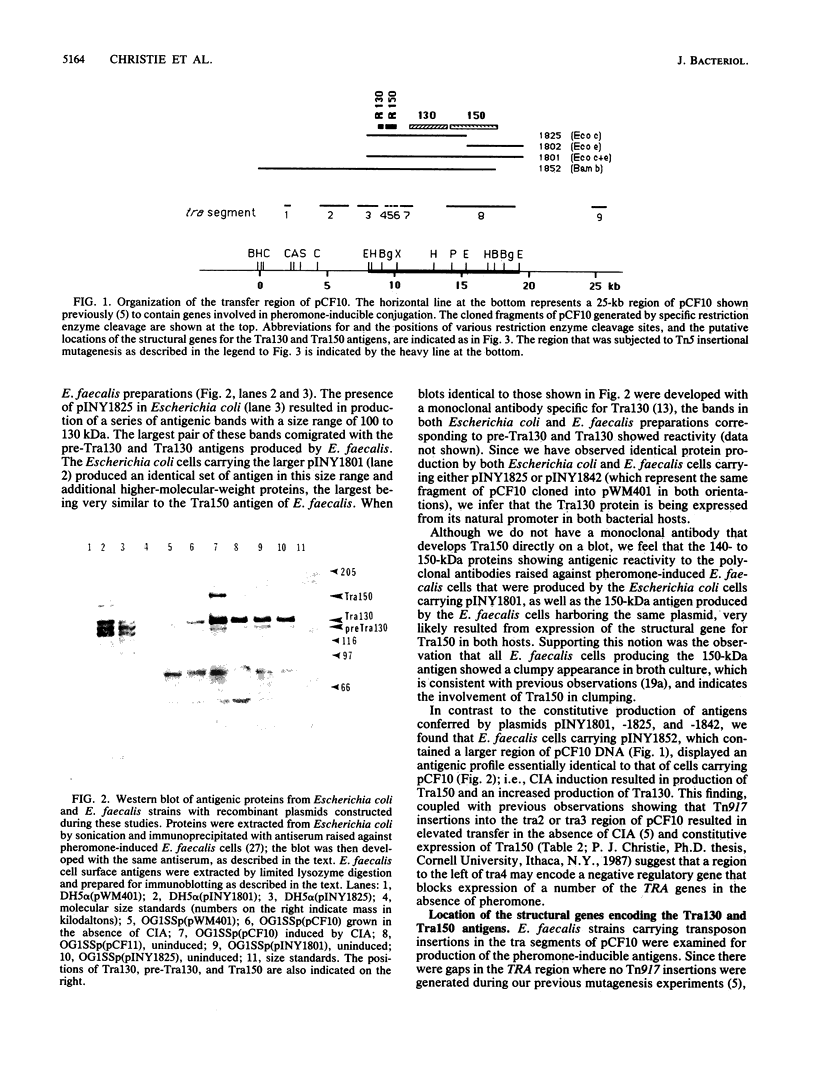
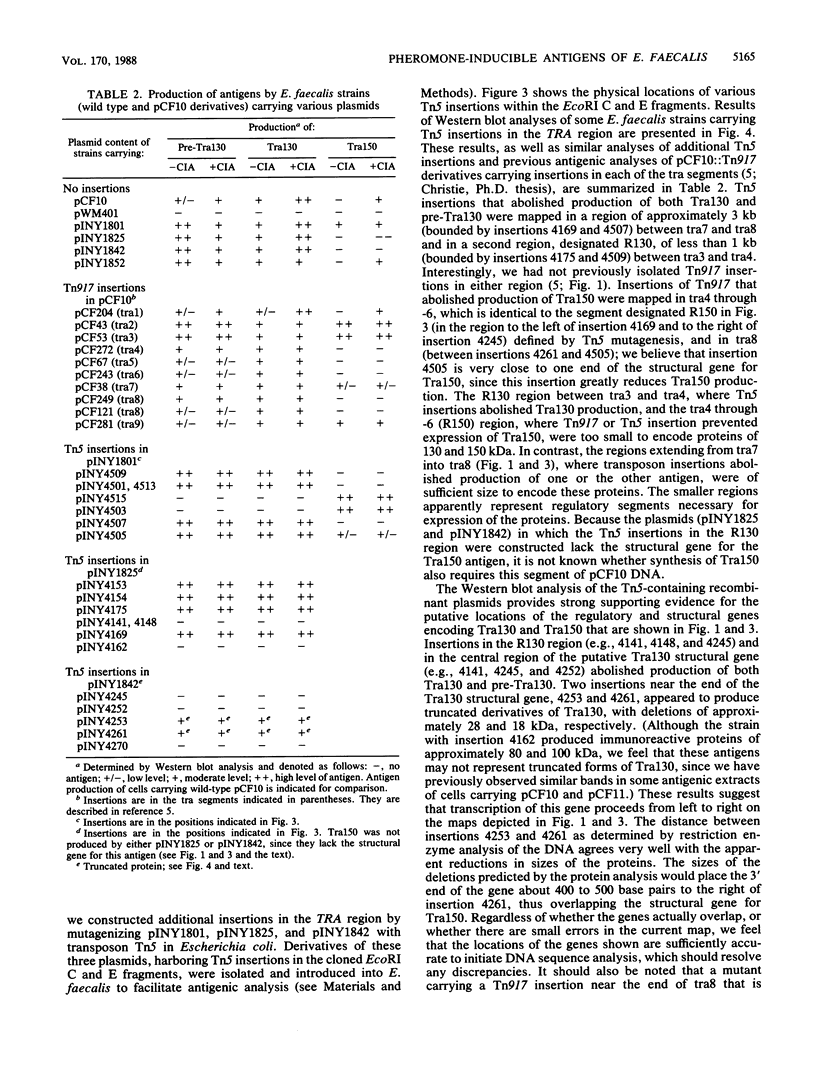
Fragments, generated by restriction enzyme digestion, of the 58-kilobase Enterococcus (Streptococcus) faecalis tetracycline resistance plasmid pCF10 were cloned and introduced into Escherichia coli and E. faecalis to characterize the pheromone-inducible conjugation system encoded by this plasmid. Western blot (immunoblot) analyses revealed that a 130-kilodalton (kDa) antigen, identical to the Tra130 antigen shown previously to be involved in pCF10-mediated pheromone-inducible surface exclusion, was produced by both bacterial hosts carrying the recombinant plasmid pINY1825 (cloned EcoRI C fragment). Both bacterial hosts carrying pINY1825 also produced various amounts of immunologically related 118- to 125-kDa antigens (designated pre-Tra130) that resembled antigens produced by E. faecalis cells carrying pCF10. An additional 150-kDa antigen, Tra150, probably involved in pheromone-induced cellular aggregation, was produced by Escherichia coli and E. faecalis hosts carrying pINY1801 (cloned EcoRI C and E fragments). The coding sequences for the Tra150 and Tra130 antigens were further localized in the TRA region of pCF10 by transposon insertion mutagenesis. Western blot analyses of the recombinant strains, and of strains carrying derivatives of pCF10 or various recombinant plasmids containing Tn5 or Tn917 insertions, suggested that the portion of pCF10 comprising the tra3 through -6 segments (previously defined by Tn917 insertional mutagenesis) contained several genes that are involved in regulating the synthesis of Tra130 and Tra150.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christie P. J., Dunny G. M. Antibiotic selection pressure resulting in multiple antibiotic resistance and localization of resistance determinants to conjugative plasmids in streptococci. J Infect Dis. 1984 Jan;149(1):74–82. doi: 10.1093/infdis/149.1.74. [DOI] [PubMed] [Google Scholar]
- Christie P. J., Dunny G. M. Identification of regions of the Streptococcus faecalis plasmid pCF-10 that encode antibiotic resistance and pheromone response functions. Plasmid. 1986 May;15(3):230–241. doi: 10.1016/0147-619x(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Christie P. J., Korman R. Z., Zahler S. A., Adsit J. C., Dunny G. M. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtilis. J Bacteriol. 1987 Jun;169(6):2529–2536. doi: 10.1128/jb.169.6.2529-2536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Zimmerman D. L., Tortorello M. L. Induction of surface exclusion (entry exclusion) by Streptococcus faecalis sex pheromones: use of monoclonal antibodies to identify an inducible surface antigen involved in the exclusion process. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8582–8586. doi: 10.1073/pnas.82.24.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G., Yuhasz M., Ehrenfeld E. Genetic and physiological analysis of conjugation in Streptococcus faecalis. J Bacteriol. 1982 Aug;151(2):855–859. doi: 10.1128/jb.151.2.855-859.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E. E., Clewell D. B. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987 Aug;169(8):3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E. E., Kessler R. E., Clewell D. B. Identification of pheromone-induced surface proteins in Streptococcus faecalis and evidence of a role for lipoteichoic acid in formation of mating aggregates. J Bacteriol. 1986 Oct;168(1):6–12. doi: 10.1128/jb.168.1.6-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984 Jun;158(3):777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Yagi Y. Identification and partial characterization of a pheromone-induced adhesive surface antigen of Streptococcus faecalis. J Bacteriol. 1983 Aug;155(2):714–721. doi: 10.1128/jb.155.2.714-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Sakagami Y., Ishii Y., Isogai A., Kitada C., Fujino M., Adsit J. C., Dunny G. M., Suzuki A. Structure of cCF10, a peptide sex pheromone which induces conjugative transfer of the Streptococcus faecalis tetracycline resistance plasmid, pCF10. J Biol Chem. 1988 Oct 5;263(28):14574–14578. [PubMed] [Google Scholar]
- Nojiri H., Takaku F., Terui Y., Miura Y., Saito M. Ganglioside GM3: an acidic membrane component that increases during macrophage-like cell differentiation can induce monocytic differentiation of human myeloid and monocytoid leukemic cell lines HL-60 and U937. Proc Natl Acad Sci U S A. 1986 Feb;83(3):782–786. doi: 10.1073/pnas.83.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorello M. L., Dunny G. M. Identification of multiple cell surface antigens associated with the sex pheromone response of Streptococcus faecalis. J Bacteriol. 1985 Apr;162(1):131–137. doi: 10.1128/jb.162.1.131-137.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorello M., Adsit J., Krug D., Antczak D., Dunny G. Monoclonal antibodies to cell surface antigens involved in sex pheromone induced mating in Streptococcus faecalis. J Gen Microbiol. 1986 Apr;132(4):857–864. doi: 10.1099/00221287-132-4-857. [DOI] [PubMed] [Google Scholar]
- Wirth R., An F. Y., Clewell D. B. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986 Mar;165(3):831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Kessler R. E., Shaw J. H., Lopatin D. E., An F., Clewell D. B. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol. 1983 Apr;129(4):1207–1215. doi: 10.1099/00221287-129-4-1207. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]