Abstract
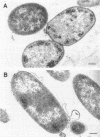
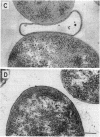
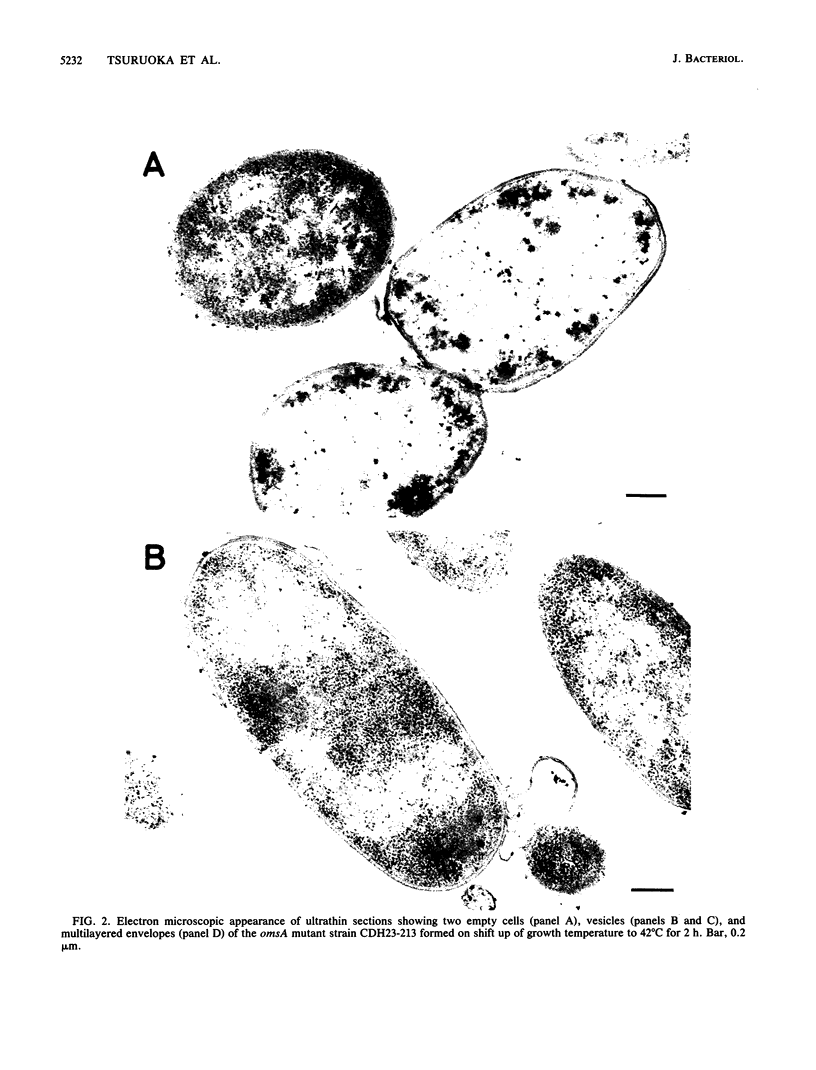
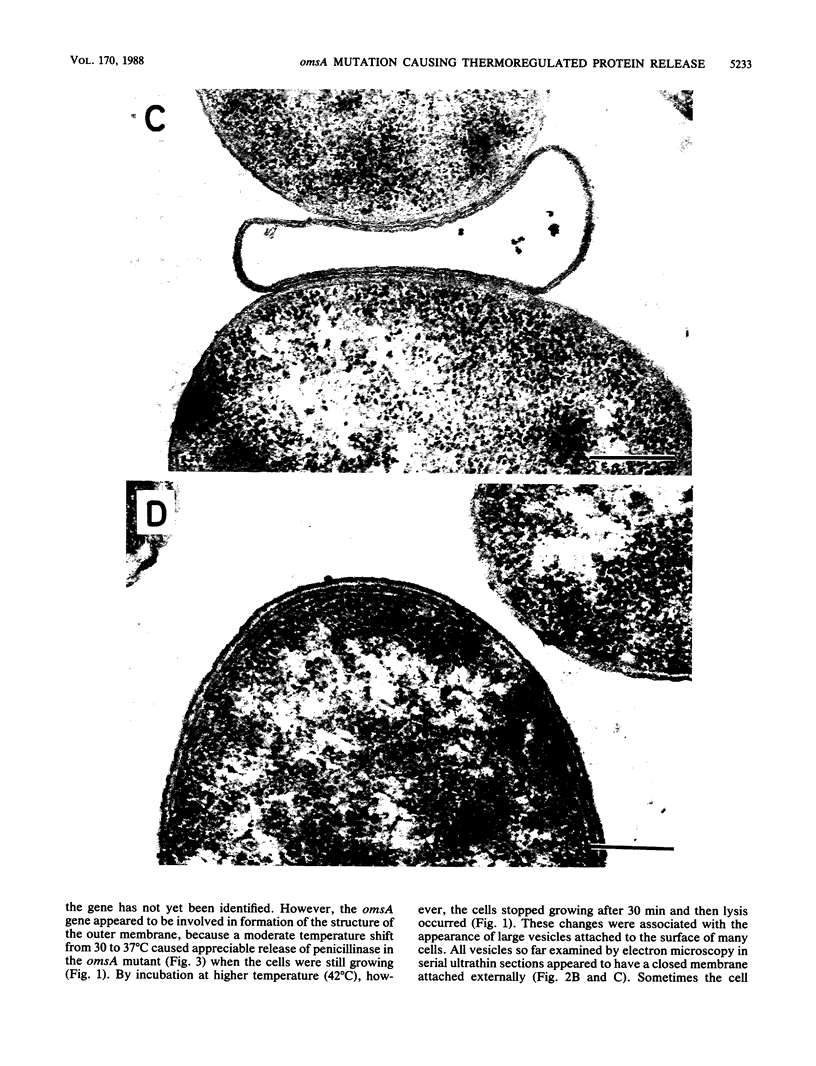
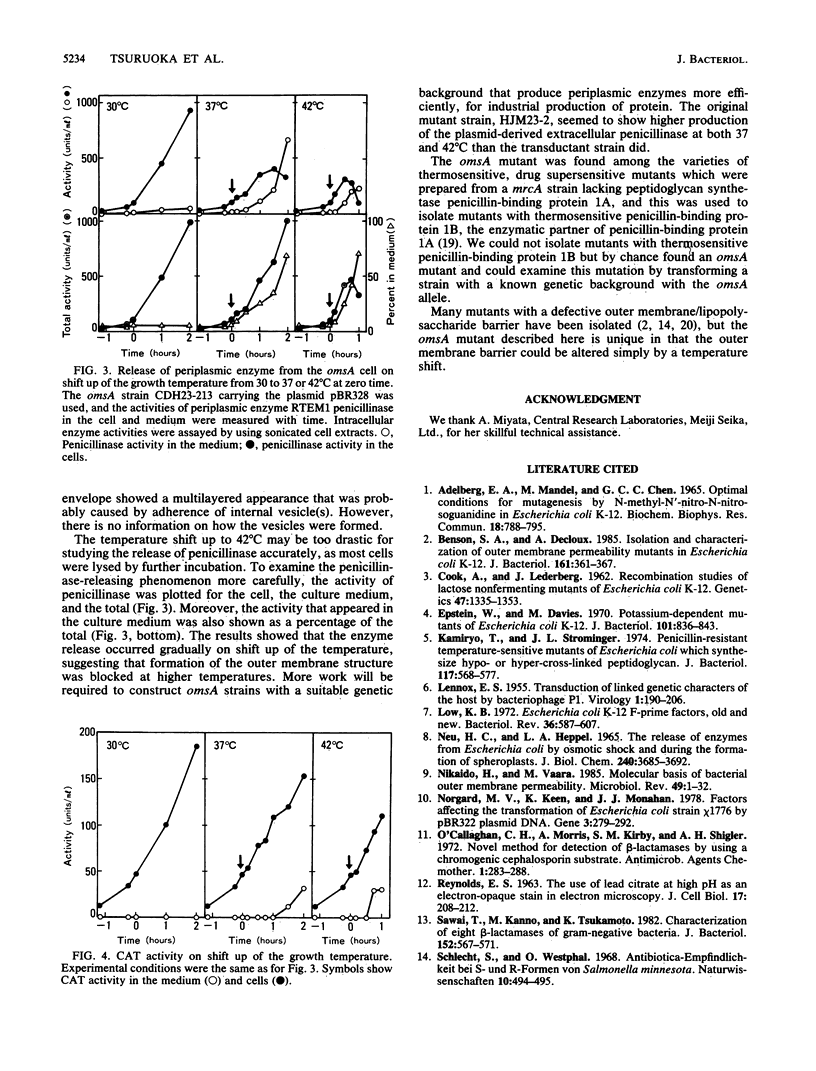
A mutant of Escherichia coli with a thermosensitive defect, possibly in the outer membrane (omsA mutant), was isolated from E. coli K-12 by mutagenization and selection for thermosensitivity and beta-lactam supersensitivity of growth. The mutant also showed very high sensitivity to other antibiotics, such as macarbomycin, midecamycin, rifampin, and bacitracin. The mutation was recessive to the wild type and was mapped at about 4 min on the E. coli chromosome between fhuA and metD. The mutation caused rapid release into the medium of periplasmic enzymes such as RTEM penicillinase but practically no cytoplasmic enzyme when cells grown at 30 degrees C were transferred to 37 or 42 degrees C. Electron microscopic observations showed many large double-layered vesicles attached to the surface of cells incubated at 42 degrees C. We conclude that the mutant had a mutation that caused a temperature-dependent defect in the outer membrane structure or its assembly (named an oms mutation). The omsA mutant may be useful for production of periplasmic proteins, which it releases into the culture medium on shift up of temperature.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson S. A., Decloux A. Isolation and characterization of outer membrane permeability mutants in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):361–367. doi: 10.1128/jb.161.1.361-367.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK A., LEDERBERG J. Recombination studies of lactose nonfermenting mutants of Escherichia coli K-12. Genetics. 1962 Oct;47:1335–1353. doi: 10.1093/genetics/47.10.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Strominger J. L. Penicillin-resistant temperature-sensitive mutants of Escherichia coli which synthesize hypo- or hyper-cross-linked peptidoglycan. J Bacteriol. 1974 Feb;117(2):568–577. doi: 10.1128/jb.117.2.568-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Kanno M., Tsukamoto K. Characterization of eight beta-lactamases of Gram-negative bacteria. J Bacteriol. 1982 Nov;152(2):567–571. doi: 10.1128/jb.152.2.567-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Antibiotica-Empfindlichkeit bei S- und R-Formen von Salmonella minnesota. Naturwissenschaften. 1968 Oct;55(10):494–495. doi: 10.1007/BF00599721. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]