Abstract
High concentrations of nitric oxide (NO) cause DNA damage and apoptosis in many cell types. Thus, regulation of NO synthase (NOS) activity is essential for minimizing effects of cytotoxic and genotoxic nitrogen oxide species. We have shown previously that NO-induced p53 protein accumulation down-regulates basal and cytokine-modulated inducible NOS (NOS2) expression in human cells in vitro. To further characterize the feedback loop between NOS2 and p53, we have investigated NO production, i.e., urinary nitrate plus nitrite excretion, and NOS2 expression in homozygous p53 knockout (KO) mice. We report here that untreated p53 KO mice excreted 70% more nitrite plus nitrate than mice with wild-type (wt) p53. NOS2 protein expression was constitutively detected in the spleen of untreated p53 KO mice, whereas it was undetectable in the spleen of wt p53 controls. Upon treatment with heat-inactivated Corynebacterium parvum, urinary nitrite plus nitrate excretion of p53 KO mice exceeded that of wt controls by approximately 200%. C. parvum treatment also induced p53 accumulation in the liver. Splenectomy reduced the NO output of C. parvum-treated p53 KO mice but not of wt p53 controls. Although NO production and NOS2 protein expression were increased similarly in KO and wt p53 mice 10 days after injection of C. parvum, NOS2 expression returned to baseline levels only in wt p53 controls while remaining up-regulated in p53 KO mice. These genetic and functional data indicate that p53 is an important transrepressor of NOS2 expression in vivo and attenuates excessive NO production in a regulatory negative feedback loop.
NO, a chemical radical, and its toxic derivatives (1–3) can cause DNA damage (4–7) and cell death (8–11) in a variety of cell types. NO also has the ability to directly modify intracellular targets such as proteins and lipid-peroxidation products (12–16). NO produces cytotoxicity at high concentrations, whereas NO at lower concentrations may have the opposite effect and protect against apoptotic cell death from various stimuli (17–19). Therefore, regulation of NO production is vital for both cell survival and genome integrity. Among the three NO synthase (NOS) isoforms, NOS1 and NOS3 are controlled by calcium fluxes and produce only nanomolar NO concentrations (20–22). NOS2 is active at resting concentrations of calcium and is capable of producing micromolar NO concentrations (23). NOS2 expression can be stimulated by cytokines (23, 24) that act by transcriptional activation of the promoter region (25–27). Functional regulatory elements that are responsive to interferon γ and lipopolysaccharide have been characterized in the first 1 kb of the murine promoter region (25, 26). In contrast, no functional elements have been identified in the proximal 1 kb of the human NOS2 promoter. In fact, a 16-kb segment of the human promoter was required to produce a response similar to the murine 1.7-kb promoter fragment (27, 28). Several inhibitors of human NOS2 expression have been identified. Glucocorticoids (29, 30) and epidermal growth factor (31) suppress mRNA synthesis, and transforming growth factor type β also reduces the stability of the NOS2 mRNA (32).
DNA damage triggers p53 protein accumulation (33, 34), which produces either growth arrest (35) or apoptosis (36, 37). Exposure of cells to high NO concentrations causes DNA damage and apoptosis, and recent results have shown that NO stimulates p53 accumulation (28, 38) and p53-mediated apoptosis (39–41). Because p53 is a transcription factor that down-regulates the promoters of bcl-2 (42) and hsp70 (43), we investigated the possibility that p53 also represses NOS2 promoter activity. We found that basal and cytokine-induced NOS2 promoter activity is down-regulated by p53 in human cells in vitro, and we have localized the region required for wild-type (wt) p53-mediated repression to approximately 400 bp upstream of the transcription start site (28).
Although the overexpression of p53 down-regulated NOS2 expression in human cells, it is unknown whether p53 transrepresses NOS2 expression in vivo. This is an important question because NO has genotoxic properties, and a loss of p53 function may promote cancer by permitting unregulated NOS2 expression and excessive NO production. To address the capacity of p53 to regulate NOS2 in vivo, we compared basal and Corynebacterium parvum-induced NO production in homozygous p53 KO mice versus wt p53 controls. We found that p53 is a regulator of basal and C. parvum-induced NOS2 expression in vivo that represses NO production in a negative regulatory feedback loop.
MATERIALS AND METHODS
C. parvum Treatment.
Six- or 7-week-old wt p53 and homozygous p53-knockout (KO) C57BL mice were purchased from Taconic Farms. The animals were kept in metabolic cages in groups of four and received a liquid diet low in nitrite and nitrate, LD = 82, purchased from Bioserv, Frenchtown, NY. The animals were treated at day 10 with a single, intraperitoneal dose, 100 mg/kg body weight, of heat-inactivated C. parvum (Burroughs Wellcome).
Urinary Nitrite plus Nitrate Excretion.
Urine samples were collected throughout the experiments; the animals were weighted in 2-day intervals. To measure urinary NO2− + NO3− concentrations, urine samples were diluted 1:10 to 1:100 in water. After NO3− was converted to NO2−, NO2− concentrations were determined with the Griess reagent (28).
NOS2 and p53 Western Analysis.
Liver and spleen protein extracts were prepared from tissue sections and homogenized on ice in RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) (46). For NOS2, 100 μg of soluble protein extract was loaded on a 7% SDS-polyacrylamide gel. To determine p53 expression, p53 protein was immunoprecipitated from 0.3 mg of total protein with 10 μl of concentrated rabbit polyclonal anti-p53 antibody CM-1 (Signet Laboratories, Dedham, MA) and 30 μl of protein A-agarose (Calbiochem). Samples were spun at 10,000 × g, and pellets were washed with RIPA buffer, heated at 95°C in loading buffer (5 Prime → 3 Prime) and separated on a 9% SDS-polyacrylamide gel. After transfer to an Immobilon-P membrane (Millipore), nonspecific binding was blocked by incubation in TBST (10 mM Tris, pH 8/150 mM NaCl/0.05% Tween 20) + 4% BSA for 4 hr at room temperature. The membranes were incubated either with a polyclonal anti-murine NOS2 antibody (Alexis Corporation, San Diego, CA), diluted 1:1,000 in TBST/2% BSA, or with the murine monoclonal anti-p53 antibody PAb 246 (Oncogene Research Products, Cambridge, MA), diluted 1:1,000 in TBST/2% BSA. Staining was performed as described (46) with anti-Ig peroxidase-coupled antibodies.
NOS2 Immunohistochemical Staining.
Tissues were fixed in 100% ethanol and paraffin-embedded. Deparaffinized and rehydrated 5-μ sections were incubated with DAKO PAP kit blocking reagents and 0.05% saponin. After washing with Tris buffer, sections were incubated with a polyclonal rabbit anti-NOS2 antibody from Transduction Laboratories (Lexington, KY), diluted 1:25 in PBS, for 1 hr. Slides were then rinsed and stained with an anti-rabbit Ig PAP kit (DAKO) as described by the manufacturer.
NOS2 Assay.
NOS2 activity was determined as described previously (46).
Statistical Analysis.
Comparisons between two characteristics were carried out by the two-tailed Student’s t test. Relationships were considered statistically significant when P < 0.05.
RESULTS
Nitrite plus Nitrate in Urine and Serum of Wt p53 and p53 KO Mice.
Basal and C. parvum-induced NO production in mice was determined as nitrite plus nitrate in serum and urine. The urinary excretion was calculated as the total amount of nitrite plus nitrate excreted per day and per kg of body weight. Both serum (Table 1) and urine levels of nitrite plus nitrate indicated that p53 KO mice have a 70% higher basal NO production than the wt p53 animals. Whereas wt p53 mice averaged 13.5 μmol/kg per day of nitrite plus nitrate excreted in urine, the p53 KO mice surpassed this level significantly (P < 0.01, two-tailed Student’s t test) by excreting an average of 23 μmol/kg per day.
Table 1.
Nitrite plus nitrate concentrations in serum of wild-type p53 and p53 knockout mice after treatment with C. parvum
| Treatment groups, n = 4–10 | Day 10 | Day 21 | Day 28 |
|---|---|---|---|
| Wt p53, control | 12 ± 3 | 16.7 ± 2 | 16.8 ± 11 |
| KO, control | 19 ± 11 | 25 ± 2.8* | 24 ± 18 |
| Wt p53, C. parvum† | 913 ± 311 | 48 ± 6.5 | 15.4 ± 1 |
| KO, C. parvum | 1,350 ± 703 | 109 ± 26* | 96 ± 37* |
All concentrations are in μM.
P < 0.05, two-tailed Student’s t test.
Inoculated at day 2.
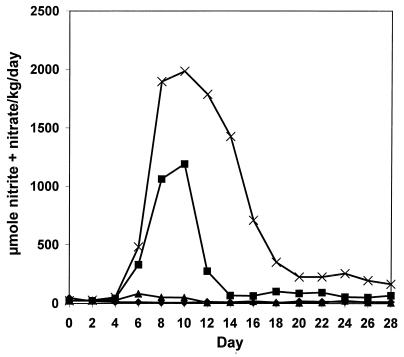
To investigate the effect of the p53 status on C. parvum-induced NO production, we treated wt p53 and homozygous p53 KO mice with heat-inactivated C. parvum, 100 mg/kg i.p., and the urinary nitrite plus nitrate excretion was measured (Fig. 1). The total urinary nitrite plus nitrate excretion of C. parvum-treated p53 KO mice was approximately three times as much as the excretion of mice with wt p53. Although urinary nitrite plus nitrate excretion of wt p53 mice returned to baseline levels after 14 days, excretion remained elevated in p53 KO mice during the 28 days of urine collection. The same trend was found by analyzing sera for nitrite plus nitrate (Table 1). Serum concentrations remained elevated in p53 KO mice and were 1.5-fold, 2.3-fold, and 6.2-fold higher at 10, 21, and 28 days after C. parvum injection, respectively, than in wt p53 controls. The expanding difference over time suggests that p53 is a feedback inhibitor of NOS2 expression in vivo.
Figure 1.
Urinary nitrite plus nitrate excretion is increased in p53 KO mice. Wt p53 (■) and p53 KO (×) mice were treated with C. parvum (100 mg/kg at day 0) or the same volume of 0.9% NaCl [wt p53 (♦), p53 KO (▴)], and daily urine samples were collected (four mice per treatment group). The total urinary nitrite plus nitrate excretion of C. parvum-treated p53 KO mice was approximately 200% higher than the output of wt p53 mice. Although urinary nitrite plus nitrate excretion of wt p53 mice returned to baseline levels after 14 days, p53 KO mice maintained an elevated excretion throughout the 28 days of urine collection.
NOS2 and p53 Expression in Liver.
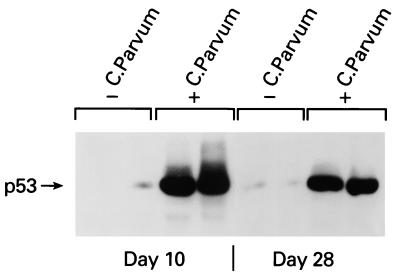
We analyzed basal and C. parvum-induced NOS2 and p53 protein accumulation in liver extracts because heat-inactivated C. parvum induces sustained hepatic NOS2 expression (44, 45). Basal expression of NOS2 protein was not detectable in either wt p53 or p53 KO mouse livers (Fig. 2A). NOS2 protein was induced markedly 10 days after C. parvum injection and declined to baseline levels only in wt p53 mice but remained elevated in liver extracts from p53 KO mice (Fig. 2A). These data indicate that the greater NO production in C. parvum-treated p53 KO mice is caused by persistent up-regulation of NOS2. At day 10 after C. parvum injection, the expression of NOS2 protein also was detectable by immunohistochemical staining (Fig. 2B), but no difference was notable between p53 wt and KO mice in the pattern of NOS2 expression in the liver and spleen. To test whether C. parvum treatment also induces p53 protein accumulation, we determined p53 protein concentrations in liver extracts by using immunoprecipitation and Western blot analysis. As shown in Fig. 3, C. parvum treatment induced p53 protein accumulation in the liver at days 10 and 28 after C. parvum injection.
Figure 2.
(A) Sustained NOS2 protein expression in liver of p53 KO mice after C. parvum treatment. Liver tissues from C. parvum-treated (100 mg/kg) wt p53 and p53 KO mice were analyzed for NOS2 expression by Western blotting (100 μg protein per lane); tissue extracts of two animals per group were analyzed. NOS2 protein levels were undetectable in controls, and C. parvum treatment produced higher levels of NOS2 protein in p53 KO mice than wt p53 mice. NOS2 protein remained markedly increased in p53 KO mice 28 days after treatment, whereas NOS2 expression returned to undetectable levels in mice with wt p53. (B) NOS2 immunostaining in the liver and spleen of wt p53 (A and C) and p53 KO (B and D) mice 10 days after i.p. injection of C. parvum (100 mg/kg). In the liver (A and B, ×400), C. parvum treatment produced cytoplasmic NOS2 staining of hepatocytes localized in focal clusters not strongly related to the lobular architecture. In addition, scattered mononuclear cells consistent with lymphocytes were stained. Cirrhosis was not evident, and there was no significant difference in damage between the wt and KO mice. Spleen (C and D, ×400): NOS2 immunostains produced intense staining of lymphocytes, which occurred predominantly in clusters distributed outside the germinal centers. Similar staining patterns occurred in both wt and KO mice. Counterstain: hematoxylin/eosin.
Figure 3.
C. parvum treatment induces wt p53 accumulation in mouse liver. Liver protein extracts of wt p53 mice were prepared 10 and 28 days after injection of C. parvum (100 mg/kg); tissue extracts of two animals per group were analyzed. p53 protein was immunoprecipitated by using 300 μg of protein extract and 10 μl of a polyclonal p53 antiserum (CM-1) and was detected with the monoclonal antibody PAb 246. p53 is highly expressed 10 and 28 days after the treatment with C. parvum.
Constitutive NOS2 Expression in the Spleen of p53 KO Mice.
Because p53 KO mice frequently develop lymphomas, we examined splenic NOS2 expression. First, we analyzed NOS2 expression in spleens from p53 KO and wt p53 mice with and without C. parvum treatment. Western blot analysis of NOS2 protein in splenic homogenates showed NOS2 protein expression in untreated p53 KO mice but not in wt p53 mice (Fig. 4). C. parvum treatment further increased NOS2 expression in the p53 KO animals as seen 21 days after the C. parvum injection, whereas NOS2 protein remained undetectable in wt p53 mice (Fig. 4). At the same time, NOS2 activity was found to be 0 and 0.5 pmol/min per mg in two splenic extracts of wt p53 mice, whereas the activities were 8.9 and 27 pmol/min per mg protein in splenic extracts from p53 KO mice.
Figure 4.
Increased splenic NOS2 expression of p53 KO mice. p53 KO and wt p53 mice were treated with C. parvum (100 mg/kg), and splenic extracts of untreated and C. parvum-treated animals were prepared 21 days later. NOS2 protein expression was measured by Western blotting (300 μg protein per lane) in two tissue extracts per treatment group. Substantial levels of NOS2 protein are expressed in p53 KO mice with or without C. parvum treatment, but it is undetectable in wt p53 mice.
To further investigate the role of the spleen in NO production, we investigated the effects of splenectomy on NO production. One week after splenectomy, p53 KO and wt p53 mice were injected with C. parvum and urinary nitrite plus nitrate levels were analyzed for 18 days. Splenectomy reduced NO production by 30–40% in the p53 KO animals, but had no effect in the wt p53 animals (Fig. 5).
Figure 5.
Splenectomy reduces C. parvum-stimulated nitrite plus nitrate excretion in p53 KO mice but not in mice with wt p53. Splenectomized and intact wt p53 (■, ♦) and p53 KO mice (×, ▴) were treated with C. parvum (100 mg/kg at day zero), and the total urinary nitrate plus nitrite excretion was measured. Splenectomy (×) reduced the urinary nitrite plus nitrate excretion of p53 KO mice by 30–40% when compared with the intact KO mice (▴). Splenectomy (■) did not affect the urinary excretion of nitrite plus nitrate in mice with wt p53 (intact = ♦). Each group contained four animals.
DISCUSSION
In vitro experiments have shown that wt p53 protein can repress NOS2 promoter activity (28), but confirmation of the in vivo relevance of this observation was lacking. Therefore, we used a p53 transgenic mouse model and tested the hypothesis that NOS2 expression is increased in p53 KO mice. Systemic NO production was monitored by measuring serum and urinary levels of nitrite and nitrate, which are stable end products of NO (47) and can be used to determine NO production in vivo (44, 45, 48). Because our previous experiments also showed that cytokine-induced NOS2 promoter activity is down-regulated by p53 (28), we used heat-inactivated C. parvum to induce NOS2 expression through the activation of the reticuloendothelial system and systemic release of cytokines (44, 45). We found that basal NO production and splenic NOS2 expression is increased in p53 KO mice, and that NO production was almost twice as high in p53 KO mice as in wt p53 controls at the peak of C. parvum induction. Although the urinary nitrite and nitrate excretion of wt p53 mice returned to baseline 14 days after C. parvum injection, the excretion continued at 5- to 6-fold higher levels in p53 KO mice for at least 28 days.
Our results show that p53 KO mice produce persistently an increased amount of NO. Basal levels of NO are generated throughout the body and participate in signal pathways of the vasculature, brain, and airways (49). These physiological NO concentrations remain low and are believed to be nontoxic. An increase of NO production above those levels is associated with pathophysiological conditions, and constitutive up-regulation of NOS2 potentially results in both cell injury and DNA damage (1, 3, 23). High NO levels in inflammation are cytotoxic toward viruses and bacteria but may also cause tissue damage and chronic inflammation (1, 23). However, the pathobiological role of NO in inflammation is controversial. Whereas excessive NO production induces cell death in many cell lines (38–41), NO also protects against apoptosis in other cell lines (50, 51) and liver damage of endotoxin-treated mice (52). NO can scavenge superoxide radicals produced by inflammation (2), and it is an inhibitor of lipid peroxidation (12). Therefore, NO production in the C. parvum model may defend against peroxide-mediated toxicity in the liver.
Homozygous p53 KO mice are cancer-prone (53), and the tumors most frequently observed are malignant lymphomas. To identify a possible pathophysiological role of NO in p53 KO mice, we next investigated major sources of uninduced and C. parvum-induced NOS2 activity. We found high levels of hepatic NOS2 protein after the injection of C. parvum, which coincided temporally with peak levels of urinary nitrite and nitrate. However, we could not detect increased liver damage in the p53 KO mice by using histology. Hepatic NOS2 protein levels declined to background levels in wt p53 mice, though NOS2 protein levels remained elevated in p53 KO mice. This observation is consistent with the hypothesis of a NOS2-p53 feedback loop, because C. parvum induced both NOS2 and p53 accumulation in wt p53 mice. p53 protein levels remained high as NOS2 protein levels fell, consistent with p53 inhibition of the NOS2 promoter. Similar patterns were observed in splenic tissues analyzed for NOS2 activity and protein expression with one exception: the p53 KO mice expressed substantial baseline levels of NOS2 protein before C. parvum stimulation. Because NOS2 protein expression was not detectable in unstimulated livers of p53 KO mice, we speculate that other factors, such as transforming growth factor type β (32), may compensate for lost p53 activity more efficiently in the liver than in the spleen. We also observed that splenectomy substantially reduced C. parvum-induced NO production in p53 KO mice but not in wt p53 controls. This observation indicates that the spleen is a major source of NOS2 activity in p53 KO mice, but not in wt p53 mice. It also extends a previous report of C. parvum-treated wt p53 mice, which found that splenic NOS2 activity was only 1.1% of the hepatic NOS2 activity (45).
Given these observations, p53 may preferentially down-regulate basal NOS2 expression in lymphocytes compared with hepatocytes, and this might contribute to the predominance of lymphomas in p53 KO mice (53). Importantly, tumor development in p53 KO mice is reminiscent of that in Li-Fraumeni patients, who have an inherited mutant p53 gene (54). The genotoxicity of NO produced by NOS2 in mice has been reported (55). Therefore, a chronically increased splenic NOS2 level in p53 KO mice may promote lymphomagenesis. A loss of p53 function may expose only some cell types, such as lymphocytes, to increased NO concentrations that are capable of overcoming the cellular defense against radical-induced DNA damage (56). Independent of a direct DNA effect, NO may also cause cancer progression by inhibiting DNA repair enzymes (2) and by its ability to induce tumor angiogenesis (57).
In summary, we present in vivo genetic and functional evidence supporting a NO-p53 regulatory loop whereby increased production of NO induces an increased level of p53 protein, which then down-regulates NOS2 expression. The data also support the hypothesis that excessive NO production in the spleen of p53 KO mice is involved in lymphomagenesis. There are multiple reports linking NO to p53 activation (28, 38–41, 58). Possible mechanisms include NO-induced DNA damage, a reduction of p53 protein degradation, or a direct modification of p53 protein by NO. Our previous studies have shown that p53 down-regulates cytokine-induced NOS2 expression and promoter activity in human and murine cells in vitro (28), and these studies of transgenic mice provide in vivo evidence supporting the existence of a NOS2 and p53 regulatory loop.
Acknowledgments
The authors thank Dorothea Dudek for her editorial assistance. We also thank Evelyn Hogg and Dr. Katherine E. Cole for their technical support and advice.
ABBREVIATIONS
- NOS
nitric oxide synthase
- KO
knockout
- wt
wild type
References
- 1.Crow J P, Beckman J S. Adv Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- 2.Wink D A, Hanbauer I, Grisham M B, Laval F, Nims R W, Laval J, Cook J, Pacelli R, Liebmann J, Krishna M, et al. Curr Top Cell Reg. 1996;34:159–187. doi: 10.1016/s0070-2137(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 3.Tamir S, Tannenbaum S R. Biochim Biophys Acta. 1996;1288:F31–F36. doi: 10.1016/0304-419x(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 4.Wink D A, Kasprzak K S, Maragos C M, Elespuru R K, Misra M, Dunams T M, Cebula T A, Koch W H, Andrews A W, Allen J S, Keefer L K. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen T, Brunson D, Crespi C L, Penman B W, Wishnok J S, Tannenbaum S R. Proc Natl Acad Sci USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.deRojas-Walker T, Tamir S, Ji H, Wishnok J S, Tannenbaum S R. Chem Res Toxicol. 1995;8:473–477. doi: 10.1021/tx00045a020. [DOI] [PubMed] [Google Scholar]
- 7.Gal A, Wogan G N. Proc Natl Acad Sci USA. 1996;93:15102–15107. doi: 10.1073/pnas.93.26.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehsel K, Kroncke K-D, Meyer K L, Huber H, Wahn V, Kolb-Bachofen V. J Immunol. 1995;155:2858–2865. [PubMed] [Google Scholar]
- 9.Geng Y J, Hellstrand K, Wennmalm A, Hansson G K. Cancer Res. 1996;56:866–874. [PubMed] [Google Scholar]
- 10.Fukuo K, Hata S, Suhara T, Nakahashi T, Shinto Y, Tsujimoto Y, Morimoto S, Ogihara T. Hypertension. 1996;27:823–826. doi: 10.1161/01.hyp.27.3.823. [DOI] [PubMed] [Google Scholar]
- 11.Melino G, Bernassola F, Knight R A, Corasaniti M T, Nistico G, Finazzi-Agro A. Nature (London) 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 12.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman B A. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 13.Kwon N S, Stuehr D J, Nathan C F. J Exp Med. 1991;174:761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimmeler S, Lottspeich F, Brune B. J Biol Chem. 1992;267:16771–16774. [PubMed] [Google Scholar]
- 15.Hentze M W, Kuhn L C. Proc Natl Acad Sci USA. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berlett B S, Friguet B, Yim M B, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:1776–1780. doi: 10.1073/pnas.93.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y M, de Vera M E, Watkins S C, Billiar T R. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- 18.Dimmeler S, Haendeler J, Nehls M, Zeiher A M. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Knethen A, Brune B. FASEB J. 1997;11:887–895. [PubMed] [Google Scholar]
- 20.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock J S, Forstermann U, Mitchell J A, Warner T D, Schmidt H H, Nakane M, Murad F. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Biochem Biophys Res Commun. 1993;193:1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- 23.Nathan C, Xie Q W. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 24.Nussler A K, Di Silvio M, Billiar T R, Hoffman R A, Geller D A, Selby R, Madariaga J, Simmons R L. J Exp Med. 1992;176:261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowenstein C J, Alley E W, Raval P, Snowman A M, Snyder S H, Russell S W, Murphy W J. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Q W, Whisnant R, Nathan C. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vera M E, Shapiro R A, Nussler A K, Mudgett J S, Simmons R L, Morris S M, Jr, Billiar T R, Geller D A. Proc Natl Acad Sci USA. 1996;93:1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forrester K, Ambs S, Lupold S E, Kapust R B, Spillare E A, Weinberg W C, Felley-Bosco E, Wang X W, Geller D A, Billiar T R, Harris C C. Proc Natl Acad Sci USA. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junquero D C, Scott-Burden T, Schini V B, Vanhoutte P M. J Physiol (London) 1992;454:451–465. doi: 10.1113/jphysiol.1992.sp019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geller D A, Nussler A K, Di Silvio M, Lowenstein C J, Shapiro R A, Wang S C, Simmons R L, Billiar T R. Proc Natl Acad Sci USA. 1993;90:522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heck D E, Laskin D L, Gardner C R, Laskin J D. J Biol Chem. 1992;267:21277–21280. [PubMed] [Google Scholar]
- 32.Vodovotz Y, Bogdan C, Paik J, Xie Q W, Nathan C. J Exp Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 34.Lu X, Lane D P. Cell. 1993;75:765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- 35.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 36.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. Nature (London) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 37.Lowe S W, Ruley H E, Jacks T, Housman D E. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 38.Messmer U K, Ankarcrona M, Nicotera P, Brune B. FEBS Lett. 1994;355:23–26. doi: 10.1016/0014-5793(94)01161-3. [DOI] [PubMed] [Google Scholar]
- 39.Fehsel K, Kroncke K D, Meyer K L, Huber H, Wahn V, Kolb-Bachofen V. J Immunol. 1995;155:2858–2865. [PubMed] [Google Scholar]
- 40.Ho Y S, Wang Y J, Lin J K. Mol Carcinogen. 1996;16:20–31. doi: 10.1002/(SICI)1098-2744(199605)16:1<20::AID-MC4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Messmer U K, Brune B. Biochem J. 1996;319:299–305. doi: 10.1042/bj3190299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyashita T, Harigai M, Hanada M, Reed J C. Cancer Res. 1994;54:3131–3135. [PubMed] [Google Scholar]
- 43.Agoff S N, Hou J, Linzer D I, Wu B. Science. 1993;259:84–87. doi: 10.1126/science.8418500. [DOI] [PubMed] [Google Scholar]
- 44.Billiar T R, Curran R D, Stuehr D J, Stadler J, Simmons R L, Murray S A. Biochem Biophys Res Commun. 1990;168:1034–1040. doi: 10.1016/0006-291x(90)91133-d. [DOI] [PubMed] [Google Scholar]
- 45.Rees D D, Cunha F Q, Assreuy J, Herman A G, Moncada S. Br J Pharmacol. 1995;114:689–693. doi: 10.1111/j.1476-5381.1995.tb17193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambs S, Merriam W G, Bennett W P, Felley-Bosco E, Ogunfusika M O, Oser S M, Klein S, Shields P G, Billiar T R, Harris C C. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 47.Lewis R S, Tamir S, Tannenbaum S R, Deen W M. J Biol Chem. 1995;270:29350–29355. doi: 10.1074/jbc.270.49.29350. [DOI] [PubMed] [Google Scholar]
- 48.Stuehr D J, Marletta M A. J Immunol. 1987;139:518–525. [PubMed] [Google Scholar]
- 49.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 50.Kim Y M, de Vera M E, Watkins S C, Billiar T R. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 51.Mannick J B, Miao X Q, Stamler J S. J Biol Chem. 1997;272:24125–24128. doi: 10.1074/jbc.272.39.24125. [DOI] [PubMed] [Google Scholar]
- 52.Billiar T R, Curran R D, Harbrecht B G, Stuehr D J, Demetris A J, Simmons R L. J Leukocyte Biol. 1990;48:565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- 53.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Butel J S, Bradley A. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 54.Malkin D, Li F P, Strong L C, Fraumeni J F, Nelson C E, Kim D H, Kassel J, Gryka M A, Bischoff F Z, Tainsky M A, et al. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 55.Gal A, Wogan G N. Proc Natl Acad Sci USA. 1996;93:15102–15107. doi: 10.1073/pnas.93.26.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambs S, Hussain S P, Harris C C. FASEB J. 1997;11:443–448. doi: 10.1096/fasebj.11.6.9194524. [DOI] [PubMed] [Google Scholar]
- 57.Jenkins D C, Charles I G, Thomsen L L, Moss D W, Holmes L S, Baylis S A, Rhodes P, Westmore K, Emson P C, Moncada S. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calmels S, Hainaut P, Ohshima H. Cancer Res. 1997;57:3365–3369. [PubMed] [Google Scholar]