Abstract
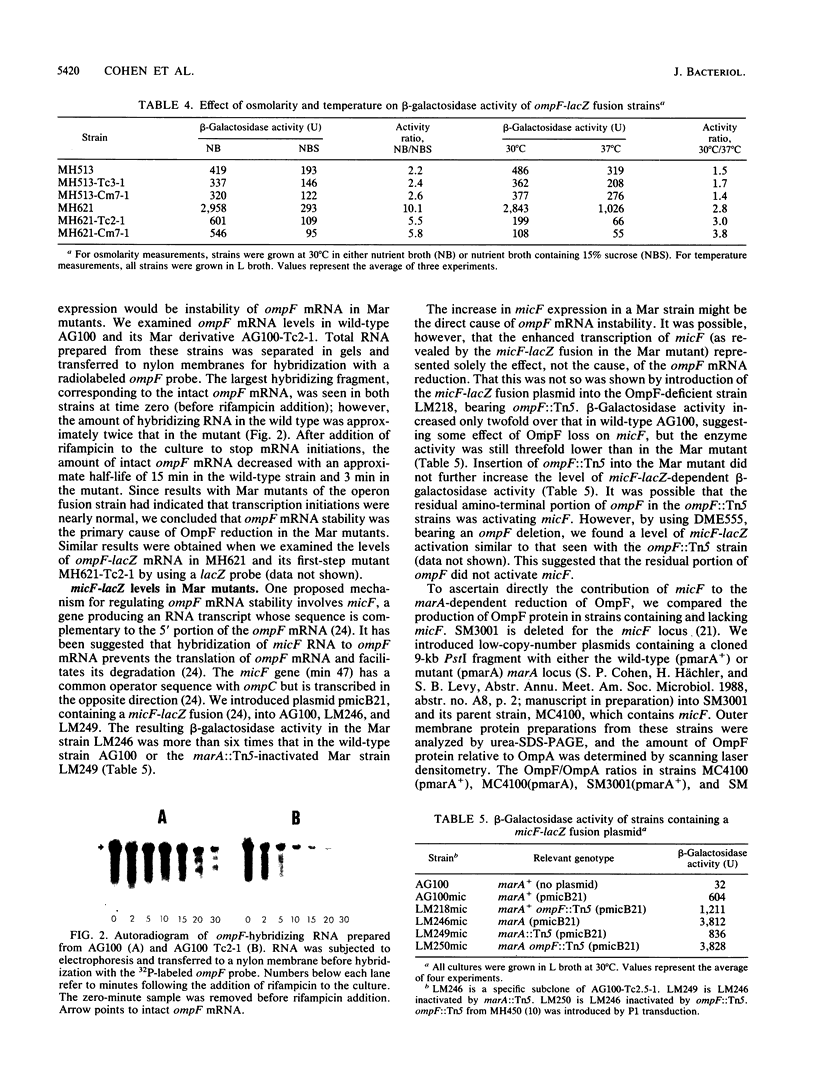
Mar (multiple antibiotic resistant) mutants of Escherichia coli express chromosomally mediated resistance to a variety of structurally unrelated hydrophilic and hydrophobic antibiotics. Insertion of transposon Tn5 into the marA locus at min 34.05 on the chromosome completely reverses the Mar phenotype (A. M. George and S. B. Levy, J. Bacteriol. 155:531-540, 1983). We found that among changes in the outer membrane of Mar mutants, porin OmpF was greatly reduced, although Mar mutants were more resistant than cells lacking only OmpF. Transduction of the marA region from a Mar strain, but not a wild-type strain, led to loss of OmpF. P1 transduction of marA::Tn5 into a Mar mutant partially restored OmpF levels. Therefore, OmpF reduction required a mutation in the marA region. Mar mutants of an ompF-lacZ operon fusion strain expressed 50 to 75% of the beta-galactosidase activity of the isogenic non-Mar parental strain, while Mar mutants of a protein fusion strain expressed less than 10% of the enzyme activity in the non-Mar strain. These changes were completely reversed by insertion of marA::Tn5. The responsiveness of OmpF-LacZ to osmolarity and temperature changes was similar in Mar and wild-type strains. Although some transcriptional control may have been present, OmpF reduction appeared to occur primarily by a posttranscriptional mechanism. The steady-state levels of ompF mRNA were twofold lower and the mRNA was five times less stable in the Mar mutant than in the wild-type strain. Expression of micF, which lowers ompF mRNA levels, was elevated in Mar strains, as revealed by a micF-lacZ fusion. Studies with strains deleted for the micF locus showed that the marA-dependent reduction of OmpF required an intact micF locus. Our findings suggest that the marA locus directly or indirectly increases micF expression, causing a posttranscriptional decrease in ompF mRNA and reduced amounts of OmpF.
Full text
PDF
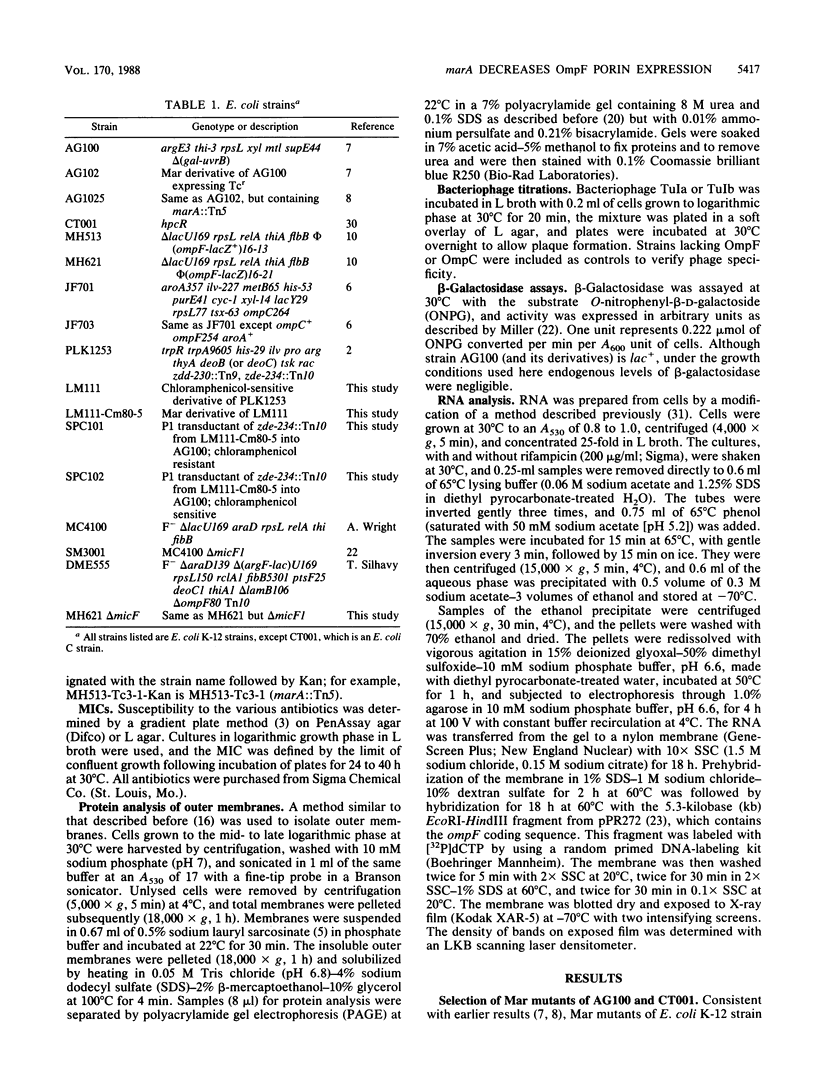


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Kato T., Hirai K., Mitsuhashi S. Norfloxacin resistance in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1987 Oct;31(10):1640–1641. doi: 10.1128/aac.31.10.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner R. M., Kuempel P. L. P1 transduction map spanning the replication terminus of Escherichia coli K12. Mol Gen Genet. 1981;184(2):208–212. doi: 10.1007/BF00272906. [DOI] [PubMed] [Google Scholar]
- Curiale M. S., Levy S. B. Two complementation groups mediate tetracycline resistance determined by Tn10. J Bacteriol. 1982 Jul;151(1):209–215. doi: 10.1128/jb.151.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Chai T. J. New major outer membrane proteins found in an Escherichia coli tolF mutant resistant to bacteriophage TuIb. J Bacteriol. 1978 Mar;133(3):1478–1483. doi: 10.1128/jb.133.3.1478-1483.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983 Aug;155(2):531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Gene in the major cotransduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J Bacteriol. 1983 Aug;155(2):541–548. doi: 10.1128/jb.155.2.541-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Transcriptional regulation of Escherichia coli K-12 major outer membrane protein 1b. J Bacteriol. 1979 Nov;140(2):342–350. doi: 10.1128/jb.140.2.342-350.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi K., Itoh M., Mizushima S. Domains involved in osmoregulation of the ompF gene in Escherichia coli. J Bacteriol. 1985 Nov;164(2):585–590. doi: 10.1128/jb.164.2.585-590.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y. L., Nara F., Ichihara S., Mizuno T., Mizushima S. Purification and characterization of the OmpR protein, a positive regulator involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1986 Nov 15;261(32):15252–15256. [PubMed] [Google Scholar]
- Kawaji H., Mizuno T., Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Lundrigan M., Earhart C. F. Reduction in three iron-regulated outer membrane proteins and protein a by the Escherichia coli K-12 perA mutation. J Bacteriol. 1981 May;146(2):804–807. doi: 10.1128/jb.146.2.804-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Mizushima S. Construction and characterization of a deletion mutant lacking micF, a proposed regulatory gene for OmpF synthesis in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1196–1202. doi: 10.1128/jb.162.3.1196-1202.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R., Reeves P. R. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987 Oct;169(10):4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Reeves P. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J Bacteriol. 1982 Jun;150(3):1016–1023. doi: 10.1128/jb.150.3.1016-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norioka S., Ramakrishnan G., Ikenaka K., Inouye M. Interaction of a transcriptional activator, OmpR, with reciprocally osmoregulated genes, ompF and ompC, of Escherichia coli. J Biol Chem. 1986 Dec 25;261(36):17113–17119. [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. J Bacteriol. 1978 Mar;133(3):1181–1189. doi: 10.1128/jb.133.3.1181-1189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C., Doherty P. Linkage relationships of two genes causing partial resistance to chloramphenicol in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1450–1451. doi: 10.1128/jb.96.4.1450-1451.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Cooper R. A. An Escherichia coli mutant defective in the NAD-dependent succinate semialdehyde dehydrogenase. Arch Microbiol. 1982 Sep;132(3):270–275. doi: 10.1007/BF00407964. [DOI] [PubMed] [Google Scholar]
- Terada M., Metafora S., Banks J., Dow L. W., Bank A., Marks P. A. Conservation of globin messenger RNA in rabbit reticulocyte monoribosomes after sodium fluoride treatment. Biochem Biophys Res Commun. 1972 May 26;47(4):766–774. doi: 10.1016/0006-291x(72)90558-x. [DOI] [PubMed] [Google Scholar]