Abstract
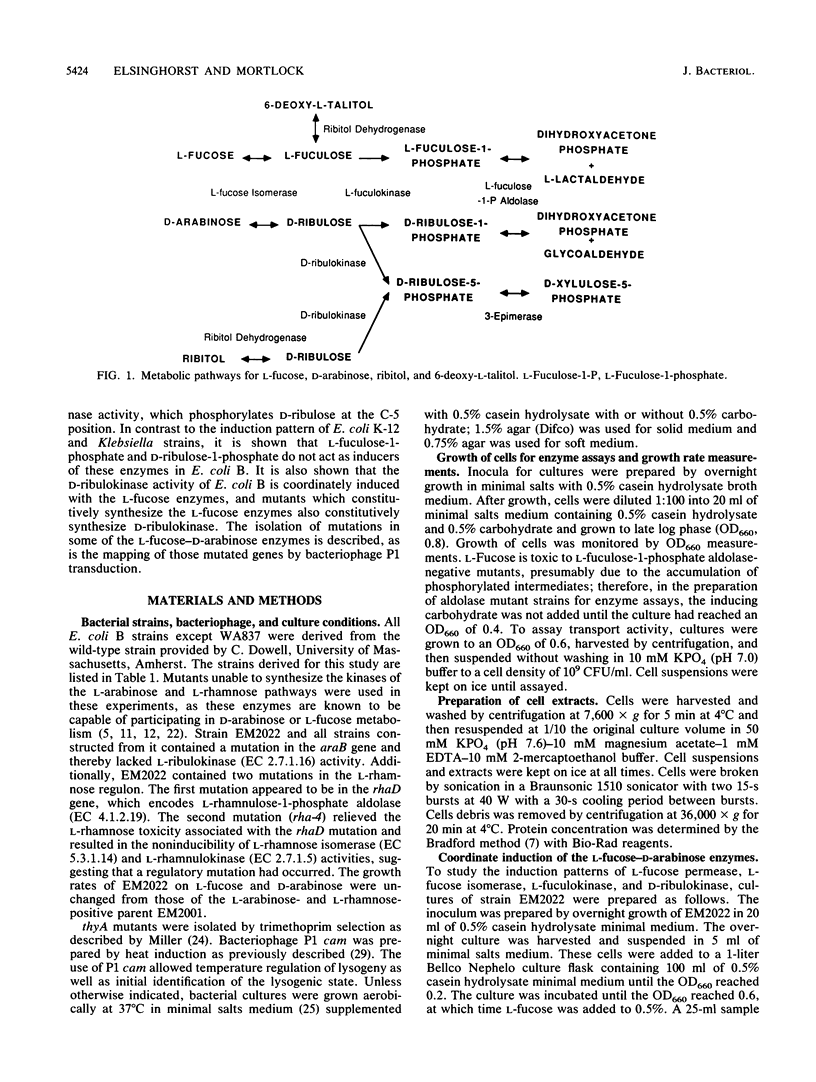
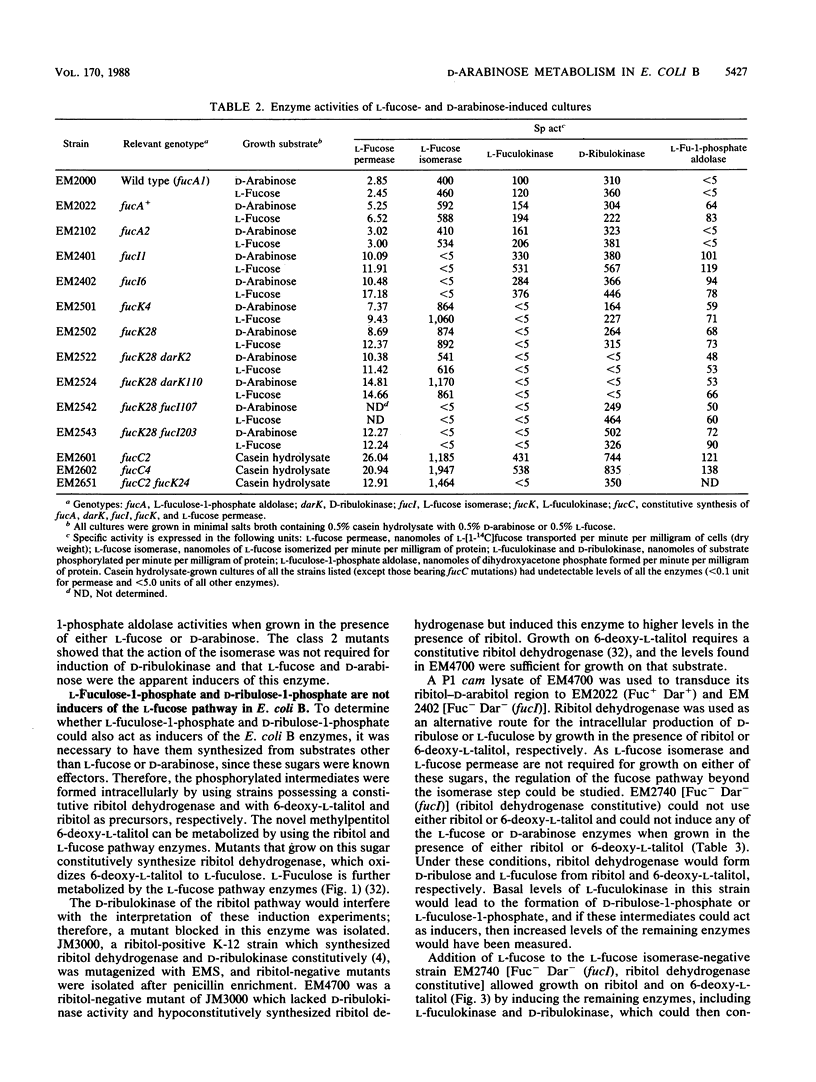
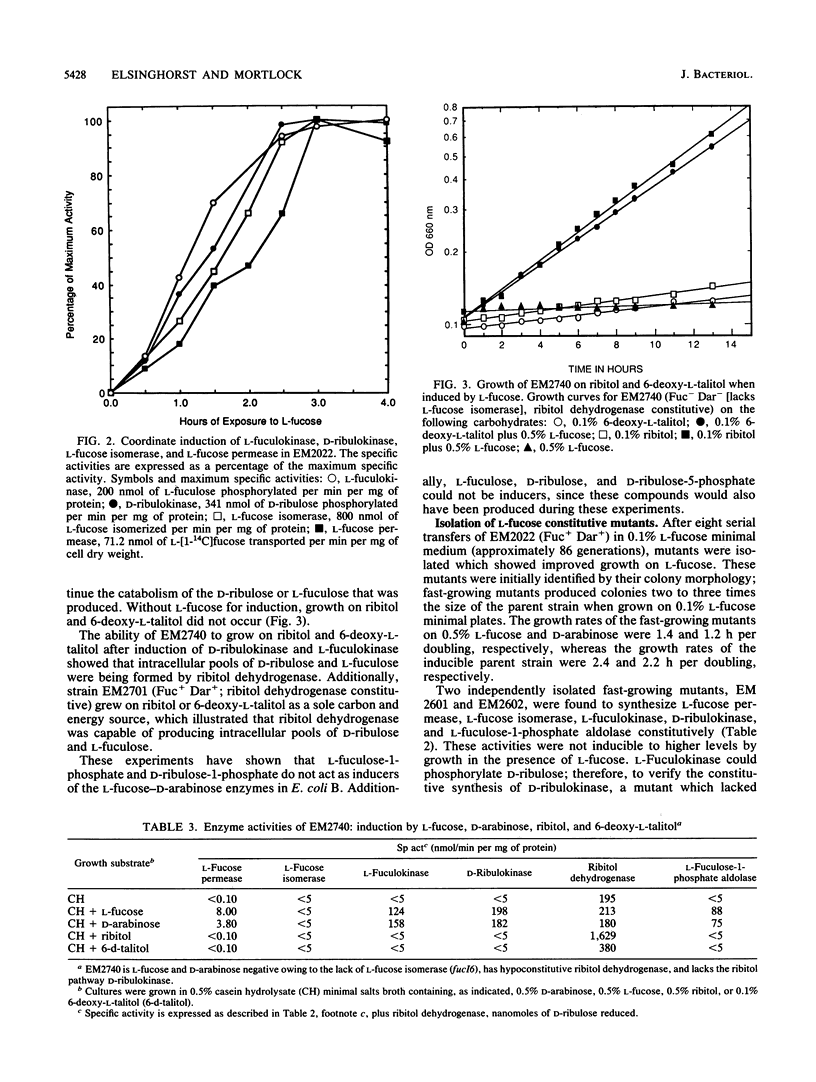
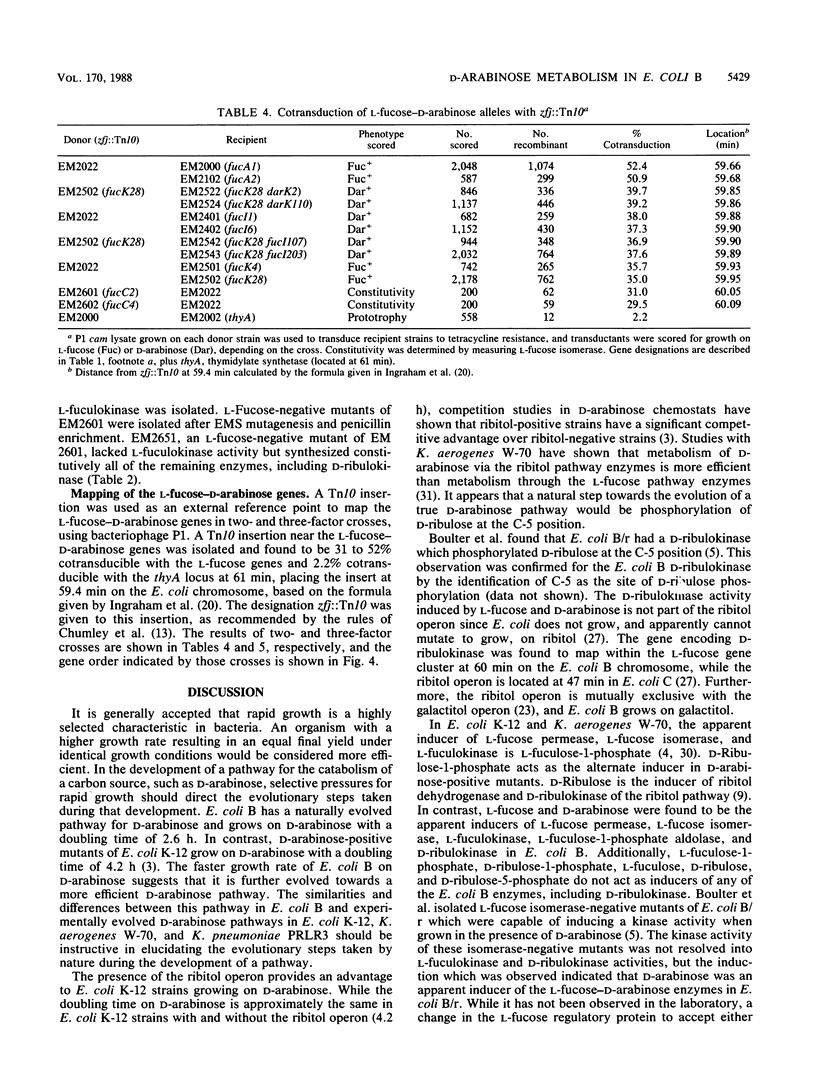
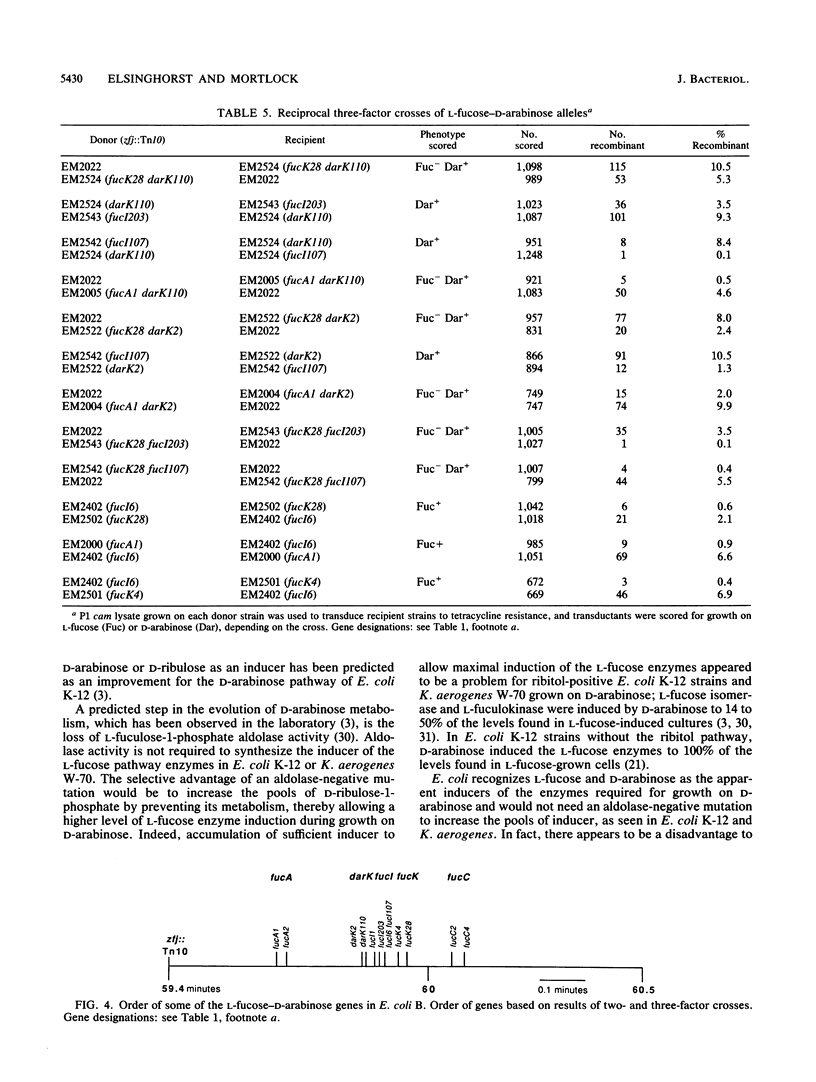
D-Arabinose is degraded by Escherichia coli B via some of the L-fucose pathway enzymes and a D-ribulokinase which is distinct from the L-fuculokinase of the L-fucose pathway. We found that L-fucose and D-arabinose acted as the apparent inducers of the enzymes needed for their degradation. These enzymes, including D-ribulokinase, appeared to be coordinately regulated, and mutants which constitutively synthesized the L-fucose enzymes also constitutively synthesized D-ribulokinase. In contrast to D-arabinose-positive mutants of E. coli K-12, in which L-fuculose-1-phosphate and D-ribulose-1-phosphate act as inducers of the L-fucose pathway, we found that these intermediates did not act as inducers in E. coli B. To further characterize the E. coli B system, some of the L-fucose-D-arabinose genes were mapped by using bacteriophage P1 transduction. A transposon Tn10 insertion near the E. coli B L-fucose regulon was used in two- and three-factor reciprocal crosses. The gene encoding D-ribulokinase, designated darK, was found to map within the L-fucose regulon, and the partial gene order was found to be Tn10-fucA-darK-fucI-fucK-thyA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON R. L., WOOD W. A. Purification and properties of L-xylulokinase. J Biol Chem. 1962 Apr;237:1029–1033. [PubMed] [Google Scholar]
- Bartkus J. M., Mortlock R. P. Construction of an improved D-arabinose pathway in Escherichia coli K-12. J Bacteriol. 1986 Mar;165(3):704–709. doi: 10.1128/jb.165.3.704-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkus J. M., Mortlock R. P. Isolation of a mutation resulting in constitutive synthesis of L-fucose catabolic enzymes. J Bacteriol. 1986 Mar;165(3):710–714. doi: 10.1128/jb.165.3.710-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J. R., Gielow W. O. Properties of D-arabinose isomerase purified from two strains of Escherichia coli. J Bacteriol. 1973 Feb;113(2):687–696. doi: 10.1128/jb.113.2.687-696.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Gielow B., McFarland M., Lee N. Metabolism of D-arabinose by Escherichia coli B-r. J Bacteriol. 1974 Feb;117(2):920–923. doi: 10.1128/jb.117.2.920-923.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHIU T. H., FEINGOLD D. S. THE PURIFICATION AND PROPERTIES OF L-RHAMNULOKINASE. Biochim Biophys Acta. 1964 Dec 23;92:489–497. doi: 10.1016/0926-6569(64)90009-4. [DOI] [PubMed] [Google Scholar]
- Chakrabarti T., Chen Y. M., Lin E. C. Clustering of genes for L-fucose dissimilation by Escherichia coli. J Bacteriol. 1984 Mar;157(3):984–986. doi: 10.1128/jb.157.3.984-986.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Ribitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):162–169. doi: 10.1128/jb.119.1.162-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Chakrabarti T., Lin E. C. Constitutive activation of L-fucose genes by an unlinked mutation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):725–729. doi: 10.1128/jb.159.2.725-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Lin E. C. Dual control of a common L-1,2-propanediol oxidoreductase by L-fucose and L-rhamnose in Escherichia coli. J Bacteriol. 1984 Mar;157(3):828–832. doi: 10.1128/jb.157.3.828-832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- HEATH E. C., GHALAMBOR M. A. The metabolism of L-fucose. I. The purification and properties of L-fuculose kinase. J Biol Chem. 1962 Aug;237:2423–2426. [PubMed] [Google Scholar]
- LeBlanc D. J., Mortlock R. P. Metabolism of D-arabinose: a new pathway in Escherichia coli. J Bacteriol. 1971 Apr;106(1):90–96. doi: 10.1128/jb.106.1.90-96.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D. J., Mortlock R. P. The metabolism of D-arabinose: alternate kinases for the phosphorylation of D-ribulose in Escherichia coli and Aerobacter aerogenes. Arch Biochem Biophys. 1972 Jun;150(2):774–781. doi: 10.1016/0003-9861(72)90097-5. [DOI] [PubMed] [Google Scholar]
- Link C. D., Reiner A. M. Genotypic exclusion: a novel relationship between the ribitol-arabitol and galactitol genes of E. coli. Mol Gen Genet. 1983;189(2):337–339. doi: 10.1007/BF00337827. [DOI] [PubMed] [Google Scholar]
- Oliver E. J., Bisson T. M., LeBlanc D. J., Mortlock R. P. D-Ribulose production by a mutant of Aerobacter aerogens. Anal Biochem. 1969 Feb;27(2):300–305. doi: 10.1016/0003-2697(69)90036-0. [DOI] [PubMed] [Google Scholar]
- Oliver E. J., Mortlock R. P. Growth of Aerobacter aerogenes on D-arabinose: origin of the enzyme activities. J Bacteriol. 1971 Oct;108(1):287–292. doi: 10.1128/jb.108.1.287-292.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Genes for ribitol and D-arabitol catabolism in Escherichia coli: their loci in C strains and absence in K-12 and B strains. J Bacteriol. 1975 Aug;123(2):530–536. doi: 10.1128/jb.123.2.530-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S., Paden C. A., Greenberg E. P. Uptake of D-xylose and D-glucose by Spirochaeta aurantia. J Bacteriol. 1984 Jul;159(1):427–428. doi: 10.1128/jb.159.1.427-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Martin E. J., Mortlock R. P. Natural and altered induction of the L-fucose catabolic enzymes in Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):91–97. doi: 10.1128/jb.127.1.91-97.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Mortlock R. P. A comparison of alternate metabolic strategies for the utilization of D-arabinose. J Mol Evol. 1977 Nov 25;10(2):111–122. doi: 10.1007/BF01751805. [DOI] [PubMed] [Google Scholar]
- St Martin E. J., Mortlock R. P. Biosynthesis and catabolism of 6-deoxy L-talitol by Klebsiella aerogenes mutants. J Bacteriol. 1980 Mar;141(3):1157–1162. doi: 10.1128/jb.141.3.1157-1162.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. A., McDONOUGH M. J., JACOBS L. B. Rihitol and D-arabitol utilization by Aerobacter aerogenes. J Biol Chem. 1961 Aug;236:2190–2195. [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]



