Abstract
Implanted biomaterials trigger acute and chronic inflammatory responses. The mechanisms involved in such acute inflammatory responses can be arbitrarily divided into phagocyte transmigration, chemotaxis, and adhesion to implant surfaces. We earlier observed that two chemokines—macrophage inflammatory protein 1α/monocyte chemoattractant protein 1—and the phagocyte integrin Mac-1 (CD11b/CD18)/surface fibrinogen interaction are, respectively, required for phagocyte chemotaxis and adherence to biomaterial surfaces. However, it is still not clear how the initial transmigration of phagocytes through the endothelial barrier into the area of the implant is triggered. Because implanted biomaterials elicit histaminic responses in the surrounding tissue, and histamine release is known to promote rapid diapedesis of inflammatory cells, we evaluated the possible role of histamine and mast cells in the recruitment of phagocytes to biomaterial implants. Using i.p. and s.c. implantation of polyethylene terephthalate disks in mice we find: (i) Extensive degranulation of mast cells, accompanied by histamine release, occurs adjacent to short-term i.p. implants. (ii) Simultaneous administration of H1 and H2 histamine receptor antagonists (pyrilamine and famotidine, respectively) greatly diminishes recruitment and adhesion of both neutrophils (<20% of control) and monocytes/macrophages (<30% of control) to implants. (iii) Congenitally mast cell-deficient mice also exhibit markedly reduced accumulation of phagocytes on both i.p. and s.c implants. (iv) Finally, mast cell reconstitution of mast cell-deficient mice restores “normal” inflammatory responses to biomaterial implants. We conclude that mast cells and their granular products, especially histamine, are important in recruitment of inflammatory cells to biomaterial implants. Improved knowledge of such responses may permit purposeful modulation of both acute and chronic inflammation affecting implanted biomaterials.
Keywords: histamine/phagocytes
The increasing usage of implanted devices in the practice of medicine demands improved knowledge of events occurring at the host/implant interface. Commonly used biomaterials may trigger an array of iatrogenic effects including inflammation, fibrosis, coagulation, and infection. In view of the inert and nontoxic nature of most biomaterials, it is puzzling that tissue contact implants very often acquire an extensive overlay of phagocytic cells (1–9). These phagocytes have been implicated in a number of subsequent adverse effects such as osteolytic changes around joint implants (7, 10, 11), stress cracking of pacemaker leads (12, 13), degradation of biomaterial implants (14, 15), and fibrosis surrounding mammary prostheses and many other types of implants (16–19).
In attempting to define the mechanisms involved in biomaterial-mediated inflammatory responses, we have somewhat arbitrarily divided the events into (i) phagocyte transmigration through the endothelial barrier, (ii) chemotaxis toward the implant, and (iii) phagocyte adherence to implant surfaces. Our earlier results indicate that interaction between the phagocyte integrin, Mac-1 (CD11b/CD18), and surface fibrinogen is critical in the adherence of phagocytes to biomaterial implants (1, 20). In addition, both macrophage inflammatory protein 1α and monocyte chemoattractant protein 1, two potent chemokines, are involved in phagocyte chemotaxis toward the implant (21). However, it is still unclear how inflammatory cells initially are recruited to the site of implantation. We hypothesized that mast cells and their granular products, specifically histamine, might play a pivotal role in this recruitment. This hypothesis was indirectly supported by three observations. First, in agreement with other investigators (22), we observe modest hyperemia and edema—typical histamine-mediated responses—surrounding experimental s.c. and i.p. implants. Second, mast cells, which store and release large amounts of histamine, often are found associated with implant surfaces (23). Third, release of histamine is known to promote transmigration of phagocytes through the endothelial barrier and up-regulation of phagocyte adhesion molecules (24–26).
Here, we report experiments aimed at testing the importance of mast cells and histamine in the pathogenesis of acute inflammatory responses to biomaterial implants. Overall, the results support the conclusion that mast cells and histamine release are critical to recruitment of inflammatory cells during biomaterial-mediated inflammatory responses.
MATERIALS AND METHODS
Materials.
Human albumin (AlbumarC 25%) was purchased from Baxter Healthcare (Glendale, CA). Polyethylene terephthalate (PET) film, type A, 0.005 mm thick, was obtained from Cadillac Plastic and Chemical (Birmingham, MI). All other reagents were purchased from Sigma.
Preparation of PET Disks.
PET film was chosen as a model biomaterial because the knitted form, Dacron (frequently used in vascular grafts), is known to provoke inflammatory and thrombotic responses (27–30). PET film was cut into circular disks of 1.2-cm diameter for use as implants. Disks then were cleaned and sterilized with multiple changes of 70% ethanol for 24 hr (detailed in ref. 1) and stored in 95% ethanol. Immediately before implantation, disks were thoroughly washed in large volumes of sterile saline to remove all traces of ethanol.
PET disks were implanted with or without precoating with either albumin (25 mg/ml) or fibrinogen (500 μg/ml) (1, 20). After 4-hr incubation at room temperature under sterile conditions, the protein-coated materials were thoroughly washed in sterile saline. It should be noted that none of the protein-coated (or uncoated) implants had detectable surface-associated endotoxin (2).
Implantation of PET Disks.
All of the animals in this study [including Swiss–Webster mice from Taconic Farms and mast cell-deficient mice (WBB6F1-W/Wv) (−/−) and congenic controls (WBB6F1-+/+) (+/+) from Jackson Laboratory] were male and 20–25 g body weight. Because inflammatory response to implanted biomaterials varies in mice of different ages or shipment batches, only mice of the same age and from a single shipment were used in individual experiments, and control groups were included in every experiment for comparison. Consequently, the experimental values shown in all graphs represent results of a single implantation experiment involving 5–6 animals per treatment group as noted. However, all experiments were repeated at least three times, and the results shown are representative of all trials.
In most cases, the variously prepared sterile PET disks were implanted i.p. as previously described (1, 2). However, in a few studies, PET disks were implanted s.c. in the dorsal area on the back of mice. The small incision then was closed with running sutures as described earlier (12). After implantation for 16 hr (earlier found to be the time of maximal phagocyte accumulation; ref. 2), both i.p. and s.c. implants were carefully recovered, and loosely bound cells were removed by gently rinsing the disks with sterile PBS.
Measurement of I.P. Histamine.
Histamine has a very short half-life (seconds to minutes in circulation) (32–34), making it impossible to measure histamine that has been released into the peritoneal space. Therefore, to assess the extent of biomaterial-mediated histamine release, we measured the amounts of residual (releasable) histamine in the peritonea of implant-bearing mice as an indirect indicator of the extent of previous histamine release. After implantation for different periods of time, mouse peritonea were instilled with cold PBS (50 mM NaPO4/100 mM NaCl, pH 7.4) to stimulate the release of histamine (35, 36). Lavage fluids were recovered and then centrifuged to remove cells. The amount of histamine in lavage fluids was determined by using a competitive ELISA from Immunotech (Westbrook, ME).
Estimation of Implant-Adherent Phagocytes.
Cytosolic and granular enzymes were released from surface-associated cells by incubation of each disk with 0.5 ml of 1.0% Triton X-100 for 1 hr. Activities of myeloperoxidase (MPO) and nonspecific esterase (NSE) in the Triton X-100 solution were used to estimate the numbers of explant-associated polymorphonuclear neutrophils (PMNs) and monocytes/macrophages (MØ), respectively. Because we earlier found that >95% of implant-associated peroxidase activities represent MPO (as opposed to eosinophil peroxidase), total peroxidase activity was taken as a measure of surface-associated PMN (1). MPO was measured spectrophotometrically at 470 nm with guaiacol as substrate (37). Control studies on purified mouse PMN indicated that the MPO activity of mouse peripheral PMN is about 23 nano units/cell. NSE is relatively restricted to MØ, and the activity of this enzyme was measured to assess the number of adherent MØ (38). NSE activity was determined by following the rate of hydrolysis of o-nitrophenyl butyrate (39) in the presence of eserine (10 mM), which will eliminate possible interference by cholinesterase (40). Enzyme assays on mouse resident peritoneal MØ showed that the NSE activity of mouse peritoneal MØ is about 11 nano units/cell. Earlier studies indicated that measured enzyme activity is a reliable measurement of the surface-associated phagocytes (for both MPO and NSE vs. cell number, r = >0.90) (1).
Phagocyte Recruitment.
We also investigated the total numbers of phagocytic cells appearing in the peritonea of implant-bearing animals. This was done by peritoneal lavage of animals after implantation for 16 hr. Mice were euthanized with inhaled CO2, and cold PBS (4 ml, pH 7.3) was injected into the peritoneal cavity. After gentle external massage of the intact peritoneal area for 2 min, the peritoneal cavity was opened, and the peritoneal fluid and disks were recovered. The cells in lavage fluids were concentrated by centrifugation (900 × g for 10 min). The cell pellets and disks then were incubated with 0.5 ml of 1.0% Triton X-100 for 1 hr to release cytosolic and granular enzymes. Enzyme assays then were conducted to estimate the numbers of phagocytes. In the results shown, the numbers of peritoneal inflammatory cells are the sum of the cells in lavage fluids and on the disks.
Administration of Histamine Antagonists and 48/80 to Mice.
In accord with earlier pharmacokinetic studies (41–44), solutions of H1 antagonist (pyrilamine, 5 mg/kg body weight) and/or H2 antagonist (famotidine, 10 mg/kg body weight), and saline (as control) were injected s.c. (supra scapular) into mice 1 hr before material samples were implanted and at 5 and 11 hr after implantation. Dosages and timing were meant to optimize the extent of continuous blockade of histamine receptors while minimizing side effects. As an additional test of the possible importance of mast cell histamine release in acute inflammatory responses to biomaterial implants, we depleted mast cell granular products (especially histamine) with multiple treatments of 48/80, a potent histamine releaser (45, 46). As established earlier (46), after five sequential i.p. injections of 48/80 (1 mg/kg body weight) at 12-hr intervals, less than 10% of peritoneal mast cells contain releasable granules.
Mast Cell-Deficient Mice.
Mast cell-deficient WBB6F1-W/Wv (−/−) mice and their normal littermates, WBB6F1-+/+ (+/+), were used when they were 3 months or older. −/− Mice have a profound deficiency in mast cells (less than 1% of the congenic +/+ controls in both peritoneum and skin) (47). These −/− mice have other abnormalities, including a macrocytic anemia and decreased numbers of granulocyte and platelet precursors in the bone marrow. Importantly, however, the numbers of phagocytes and platelets in peripheral blood of adult −/− mice are normal (48, 49). A number of earlier investigations have shown these mice to be a valuable tool for determination of mast cell functions in vivo (e.g., refs. 50–55).
Reconstitution of Mast Cells in Mast Cell-Deficient Mice.
Bone marrow cells were cultured from femurs of control animals (+/+) for 3 weeks in WEHI-3-conditioned medium as described earlier (54). Mast cells (>98% purity) were injected i.p. into −/− mice at 1 × 106 per animal. The mast cell-repleted animals (W/Wv+MC) were kept for 7 weeks before implantation studies. Others have shown that the majority of transferred mast cells survive well beyond 7 weeks (56).
RESULTS
Implanted Biomaterials Trigger the Release of Histamine.
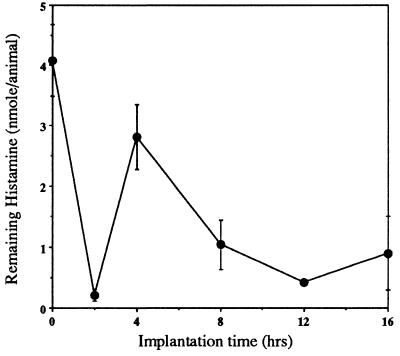
To define the mechanism(s) governing biomaterial-mediated phagocyte recruitment, we hypothesized that the release of histamine might be involved. To test this hypothesis, we first determined whether the presence of biomaterial implants might stimulate histamine release, thereby decreasing the amounts of releasable histamine. Indeed, the amounts of histamine released from surrounding tissues by cold PBS lavage decrease profoundly immediately after implantation (Fig. 1). Interestingly, these depressed levels of releasable histamine rebound at 4 hr after implantation. Inasmuch as our control experiments indicate that recruitment of inflammatory cells to the peritoneal cavity commences ≈4 hr after implantation, these newly recruited naive cells (possibly mast cells and MØ) may contribute to the observed rebound in i.p. releasable histamine.
Figure 1.
The amounts of releasable histamine remaining in the peritonea of control (time “0”) and implant-bearing mice. After implantation with PET disks for different periods of time, mice were euthanized and the peritoneal cavity was instilled with cold sterile PBS. Histamine concentrations in cell-free lavage fluids were assayed as described in Materials and Methods. Vertical lines denote ± 1 SD (n = 5 in all cases).
Histamine Receptor Antagonists Suppress the Accumulation of Phagocytes on Implant Surfaces.
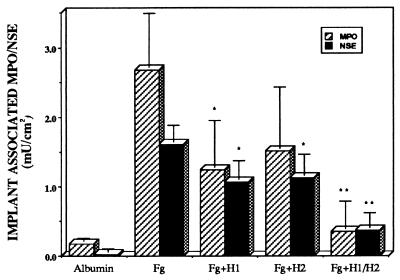
To investigate the possible role of histamine in phagocyte recruitment and, subsequently, adhesion to implants, Swiss–Webster mice were repetitively injected with histamine receptor antagonists for either H1 or H2 receptors or saline (as control) before and after implantation. Neither pyrilamine nor famotidine, when administered individually, had significant effect on the accumulation of inflammatory cells on implant surfaces (Fig. 2). However, administration of a combination of H1 and H2 antagonists almost completely blocked accumulation of both PMN and MØ on implant surfaces (Fig. 2). The relative absence of implant-adherent phagocytes in animals treated with combined H1+H2 blockers might reflect either decreased immigration of phagocytes into the peritoneal cavity or some more discrete effect of the histamine antagonists on the ability of phagocytes to adhere to implant surfaces. We carried out limited qualitative studies to test whether histamine receptor antagonists might block the binding of (phorbol) activated phagocytes (having up-regulated Mac-1) to biomaterial surfaces. Our results indicate that activated human PMN and activated mouse resident peritoneal MØ bind equally well to fibrinogen-coated PET surfaces in the presence or absence of H1+H2 receptor antagonists (data not shown).
Figure 2.
Accumulation of PMN and MØ on the surfaces of albumin- or fibrinogen (Fg)-coated PET implants after 16 hr in vivo. Implants were placed in Swiss–Webster mice, some of which also were treated with pyrilamine (H1), famotidine (H2), or both as described under Materials and Methods. Values shown represent total implant-associated MPO (crosshatched bars) and NSE (dark stippled bars) activities. Vertical lines denote ± 1 SD (n = 5 in all cases). Significance of differences between H1/H2 treated animals vs. untreated mice with Fg-coated implants: ∗, P < 0.05; ∗∗, P < 0.01. As estimated by MPO activity, numbers of surface-associated PMN in control mice were 783 ± 1,739/cm2 on albumin-coated disks, 116,500 ± 33,900/cm2 on Fg-coated disks in untreated animals, 53,900 ± 29,600/cm2 on Fg-coated disks in H1-treated animals, 66,100 ± 38,300/cm2 on Fg-coated disks in H2-treated animals, and 15,400 ± 17,300/cm2 on Fg-coated disks in animals treated with the combination of H1+H2 antagonists. Estimated numbers of MØ were 2,550 ± 3,910/cm2 on albumin-coated disks, 146,400 ± 21,800/cm2 on Fg-coated disks in untreated animals, 97,300 ± 24,500/cm2 on Fg-coated disks in H1-treated animals, 102,700 ± 27,300/cm2 on Fg-coated disks in H2-treated animals, and 33,600 ± 19,100/cm2 on Fg-coated disks in animals treated with the combination of H1+H2 antagonists.
Histamine Release Is Required for Recruitment of Phagocytes to Peritoneal Implants.
The possibility that histamine might be important in the initial recruitment of inflammatory cells to the implant site (perhaps by up-regulating endothelial P-selectin expression; refs. 57 and 58) was directly assessed by counting total numbers of phagocytes appearing in the peritonea and on the implants of variously treated animals. Indeed, 16 hr after implantation of fibrinogen-coated material, mice treated with a combination of H1+H2 blockers had far fewer peritoneal phagocytes of both types (Table 1). Therefore, release of histamine may trigger the initial recruitment of inflammatory cells to the peritoneal cavity, and histamine receptor blockade decreases the numbers of implant-adherent phagocytes largely by preventing their initial appearance at the implantation site. However, these results left unresolved the question of which cell type(s) might be responsible for implant-mediated histamine release.
Table 1.
Total numbers of inflammatory cells (implant surfaces + peritoneal lavage) within the peritonea of control mice, sham-operated mice (operated without placement of an implant), and mice bearing fibrinogen-coated PET implants, variously treated with saline, H1, H2, and the combination of H1 + H2 antagonists
| MPO, milliunits/mouse | Estimated PMN, ×106 | NSE, milliunits/mouse | Estimated MØ, ×104 | |
|---|---|---|---|---|
| Implant + saline | 96.5 ± 21.6 | 4.20 ± 0.93 | 27.9 ± 3.1 | 2.54 ± 0.28 |
| Implant + H1 | 90.7 ± 12.9 | 3.94 ± 0.56 | 20.9 ± 2.4** | 1.90 ± 0.22** |
| Implant + H2 | 65.5 ± 14.9 | 2.85 ± 0.65 | 23.0 ± 4.2 | 2.09 ± 0.38 |
| Implant + H1/H2 | 37.7 ± 12.9** | 1.64 ± 0.56** | 16.6 ± 5.8* | 1.51 ± 0.53* |
| Sham operated | 18.13 ± 6.00** | 0.79 ± 0.26** | 10.8 ± 2.3** | 0.98 ± 0.21** |
| Control (no surgery) | 0.027 ± 0.053** | 0.001 ± 0.002** | 10.8 ± 1.5** | 0.98 ± 0.14** |
MPO and NSE activities were used to estimate the numbers of PMN and MØ, respectively. Values represent the mean ± 1 SD (n = 5 in all cases). Significance of differences vs. untreated animals bearing fibrinogen-coated implants (implant + saline): ∗, P < 0.05; ∗∗, P < 0.01.
Mast Cells Are the Most Important Effectors of Phagocyte Recruitment to Implants.
Both mast cells and MØ can produce and release histamine (59–61). However, relatively large numbers of mast cells are present in the peritoneum (2–5% of total cells) (23), and peritoneal mast cells may contain 2–10 picograms of histamine per cell (62). On the other hand, MØ only contain small amounts of histamine (about 0.5% of the histamine stored in mast cells) (35, 61, 63). Therefore, mast cells appeared to be the most likely source of the histamine responsible for implant-mediated phagocyte recruitment. Indeed, in a preliminary study, repetitive administration of 48/80 depleted >90% of histamine stores from peritoneal mast cells (45, 46). We found that phagocyte recruitment to the peritoneum is also greatly decreased—<20% of normal PMN accumulation and <50% of normal MØ accumulation on implant surfaces (as compared with animals repetitively injected with saline) (results not shown).
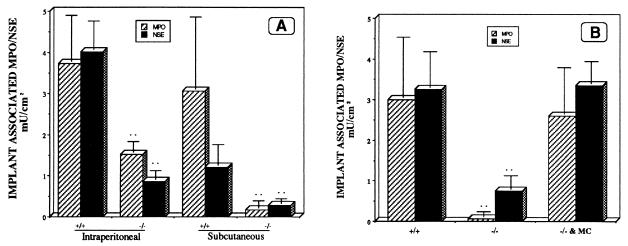
As a more direct test of the possible role of mast cells in biomaterial-mediated inflammatory responses, we used −/− mice and normal congenic, +/+, animals. −/− Mice have a distinctly blunted inflammatory response to implanted biomaterials compared with their +/+ controls (<40% of normal PMN accumulation and <20% of normal MØ accumulation on i.p. implants) (Fig. 3A). Because inflammatory responses to foreign bodies may vary markedly with site of implantation, we also carried out limited parallel studies on these animals with s.c. implants. As was the case with i.p. implants, after 16-hr implantation the s.c. implants were found to have far fewer adherent phagocytes than did matched normal animals (Fig. 3A). The possible role of mast cells in biomaterial-mediated inflammatory responses was further tested by using mast cell reconstitution of −/− mice. As expected, replenishment of mast cells restores “normal” inflammatory responses to biomaterial implants in mast cell-deficient mice (Fig. 3B).
Figure 3.
Accumulation of PMN and MØ on the surfaces of fibrinogen-coated PET implants after 16-hr implantation in mast cell-deficient mice (−/−) or otherwise congenic normal animals (+/+). Values shown represent implant-associated MPO (crosshatched bars) and NSE (dark stippled bars) enzyme activities. Vertical lines denote ± 1 SD (n = 5 in all cases). Significance of differences vs. control mice, ∗∗: P < 0.01. (A) On i.p. implants, estimated numbers of PMN calculated from MPO activities were 162,200 ± 48,700/cm2 in +/+ and 66,500 ± 11,300/cm2 in −/− mice. Estimated numbers of MØ were 365,500 ± 62,700/cm2 in +/+ and 79,100 ± 19,100/cm2 in −/− mice. On s.c. implants, estimated numbers of PMN were 133,500 ± 76,100/cm2 in +/+ and 7,390 ± 7,830/cm2 in −/− mice. Estimated numbers of MØ were 110,900 ± 44,500/cm2 in +/+ and 25,500 ± 10,900/cm2 in −/− mice. (B) Estimated numbers of PMN (cells/cm2) calculated from MPO activities were 130,400 ± 64,300 in +/+, 2,600 ± 5,700 in −/−, and 113,000 ± 49,600 in mast cell-reconstituted mast cell-deficient mice (−/− & MC). Estimated numbers of MØ (cells/cm2) calculated from NSE activities were 295,000 ± 80,000 in +/+, 68,500 ± 30,000 in −/− mice, and 305,000 ± 50,000 in mast cell-reconstituted mast cell-deficient mice.
Mast Cells Adjacent Implants Are Degranulated.
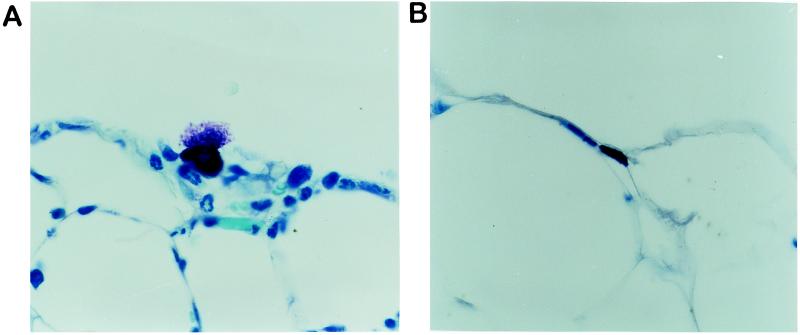
Mesothelial specimens were obtained both from the omentum immediately adjacent to PET implants and from distant sites with no contact with the implant surface. As shown in Fig. 4, there was extensive degranulation of mast cells in close proximity to the biomaterial implants (Fig. 4A). However, mast cells in areas distant from the implant or from (sham operated) control animals showed no visible degranulation (Fig. 4B).
Figure 4.
Sections of omentum from an area immediately overlying a 16-hr PET i.p. implant (A) and from a distant site in the same animal that had no contact with the implant (B). These preparations were stained with toluidine blue, which permits the visualization of mast cell granules. Magnification, ×120.
DISCUSSION
Only a few hours after implantation, most biomaterial implants prompt some degree of acute inflammatory responses as reflected by the accumulation of inflammatory cells. The mechanisms of such foreign body-mediated inflammatory responses are largely undetermined. Based on many earlier observations, the mechanisms involved in these responses can be arbitrarily divided into three consecutive events: (i) phagocyte transmigration through the endothelial barrier, (ii) chemotaxis toward the implant, and, finally, (iii) adherence to the biomaterial. Our earlier results have shown that interaction between the phagocyte integrin, Mac-1 (CD11b/CD18), and one short peptide displayed by surface-adsorbed fibrinogen (P1) is absolutely required for phagocyte adherence to biomaterial implants (1, 20). Furthermore, both macrophage inflammatory protein 1α and monocyte chemoattractant protein 1 have been shown to participate in the chemotaxis of phagocytes toward biomaterial implants (21). However, the processes involved in the critical initial step—phagocyte transmigration through the endothelial barrier—remain undefined.
For a number of reasons, we hypothesized that release of histamine might be important for the accumulation of inflammatory cells on implant surfaces. We and others (22) have observed that the tissue surrounding biomaterial implants is often hyperemic and edematous, features of classical histaminic responses. Not only does direct administration of histamine lead to vasodilation, increased vascular permeability, and localized edema, but it is well known that histamine receptor antagonists can reduce or prevent the hyperemia and edema associated with histamine release (64–68).
The proposition that acute inflammatory responses mediated by biomaterial implants might involve histamine is supported by our observation that the presence of biomaterial implants depletes the content of releasable histamine in peritonea of implant-bearing animals. To dissect the importance of histamine per se, we attempted to block possible histamine effects by administration of histamine receptor antagonists—pyrilamine (H1 receptor antagonist) and famotidine (H2 receptor antagonist). We first suspected that histamine might foster recruitment of inflammatory cells and increase albumin leakage in postcapillary venules via effects on H1 receptors, particularly those on endothelial cells. Indeed, several earlier studies (57, 58, 69) indicate that histamine exerts its action on capillary permeability at the venular level via H1 receptors. Histamine also up-regulates the expression of P-selectin on the endothelial cell surface, thereby enhancing the “rolling” of passing phagocytes and supporting transient leukocyte adhesion to endothelial cells (58, 70, 71). Surprisingly, neither H1 nor H2 receptor antagonists significantly diminish the accumulation of inflammatory cells on implant surfaces.
However, a few earlier observations in both humans and animals have shown that the combination of H1- and H2-receptor antagonists is more effective than either antagonist alone in reducing histamine-mediated inflammatory responses (particularly vasodilation) (71–73). Specifically, histamine-mediated vascular smooth muscle responses, especially changes of capillary pressure and transcapillary exchange of albumin, have been found to involve both H1 and H2 receptors (41, 74, 75). We therefore treated animals simultaneously with H1 and H2 blockers and found that phagocyte accumulation on peritoneal implants was almost totally prevented. Nonetheless, it was still not clear whether histamine was involved in recruitment of inflammatory cells to implant sites, adhesion of inflammatory cells to implant surfaces, or both. Enumeration of both implant-adherent and total i.p. phagocytes indicates that treatment with histamine receptor antagonists reduces the total accumulation of inflammatory cells. Because H1 and H2 receptor antagonists do not inhibit the binding of (phorbol) activated PMN and MØ to fibrinogen-coated surfaces, we tentatively conclude that the combined antihistamines are probably preventing the initial recruitment of phagocytic cells to the peritoneum.
Further experiments indicate that, not unexpectedly, mast cells are the predominant source of histamine release. Both mast cells and MØ may produce and release histamine (59–61). However, the peritoneal space contains relatively large numbers of mast cells (2–5% of total lavage cells) (23, 76). Furthermore, MØ can only release small amounts of histamine (about 0.5% of the amount of histamine that can be released by mast cells on a per-cell basis) (35, 61, 63). Therefore, we suspected that mast cells might be primarily responsible for the release of histamine and subsequent triggering of the recruitment of inflammatory cells to implant sites. Indeed, when histamine-containing mast cell granules are depleted (by repetitive administration of 48/80) before implants are placed, far fewer inflammatory cells are recruited to the peritoneal cavity and appear on the implant surface.
However, in the case of pharmacologic interventions, there is always the possibility for artifact caused by secondary, unknown effects of particular drugs. We therefore further tested the possible importance of mast cell histamine release by using mast cell-deficient mice (−/−) and their normal littermates (+/+). After i.p. implantation for 16 hr, far fewer PMN (<40% of normal) and MØ (<20% of normal) accumulated on implant surfaces in mast cell-deficient mice than in their congenic controls. As expected, the reduction in phagocyte numbers adherent to implants also is associated with significant decreases in the numbers of inflammatory cells recruited to the peritonea. Being aware that −/− mice might have attendant abnormalities that could influence acute inflammatory responses, we tested the importance of mast cells per se by reconstituting the mast cell population in previously mast cell-deficient mice. In support of our hypothesis, we observed full restoration of acute inflammatory responses (reflected by the extent of phagocyte recruitment and adhesion) to biomaterial implants in mast cell reconstituted animals. Furthermore, similar studies on s.c. implants yielded similar results.
These results provide evidence that biomaterial-mediated acute inflammatory responses are both histamine and mast cell dependent. Unfortunately, we still do not know the mechanism(s) through which biomaterial implants actually trigger mast cell release reactions. However, based on these results and many earlier studies, the possible mechanism of biomaterial-mediated phagocyte recruitment can be summarized as follows: (i) Shortly after implantation, biomaterials somehow trigger the activation of mast cells that release granular products, including histamine. (ii) Free histamine then prompts both hyperemia and enhanced expression of endothelial adhesion molecules such as P-selectin. (iii) Up-regulation of endothelial adhesion molecules enhances the arrest and diapedesis of phagocytic cells through the endothelial barrier. (iv) Once within the general area of the implant, incoming phagocytic cells are directed toward the implant surface by the chemokines macrophage inflammatory protein 1α and monocyte chemoattractant protein 1. Finally, adherence of the incoming phagocytes to the implant surface occurs, at least in part, through interactions between phagocyte Mac-1 and one epitope on spontaneously adsorbed fibrinogen. Although many other intermediate steps may remain unknown, we are confident that a more comprehensive understanding of the sequence of events leading to biomaterial-mediated inflammatory responses may permit the future rational design of more biocompatible implantable and blood-contact biomaterials.
Acknowledgments
We thank Dr. Lindsay Hough for valuable discussions and Diane Konzen for expert manuscript preparation. This work was supported by grants from the American Heart Association and National Institutes of Health.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PMN, polymorphonuclear neutrophils; MPO, myeloperoxidase; MØ, monocytes/macrophages; NSE, nonspecific esterase; PET, polyethylene terephthalate.
References
- 1.Tang L, Eaton J W. J Exp Med. 1993;178:2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang L, Lucas A H, Eaton J W. J Lab Clin Med. 1993;122:292–300. [PubMed] [Google Scholar]
- 3.Marchant R E, Miller K M, Anderson J M. J Biomed Mater Res. 1984;18:1169–1190. doi: 10.1002/jbm.820180917. [DOI] [PubMed] [Google Scholar]
- 4.Kossovsky N, Heggers J P, Parsons R W, Robson M C. Plast Reconstr Surg. 1983;71:795–802. doi: 10.1097/00006534-198306000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Copeland M, Kressel A, Spiera H, Hermann G, Bleiweiss I J. Plast Reconstr Surg. 1993;92:1179–1181. doi: 10.1097/00006534-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Cook P D, Osborne B M, Connor R L, Strauss J F. Am J Surg Pathol. 1995;19:712–717. doi: 10.1097/00000478-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Athanasou N A, Quinn J, Bulstrode C J K. J Bone Joint Surg. 1992;74:57–62. doi: 10.1302/0301-620X.74B1.1732267. [DOI] [PubMed] [Google Scholar]
- 8.Santavirta S, Konttinen Y T, Bergroth V, Eskola A, Tallroth K, Lindholm S. J Bone Joint Surg. 1990;72:252–258. [PubMed] [Google Scholar]
- 9.Jiranek W A, Machado M, Jasty M, Jevaevar D, Wolfe H J, Goldring S R, Goldberg M J, Harris W H. J Bone Joint Surg. 1993;75:863–879. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Holtrop M E, Cox K A, Glowacki J. Calcif Tissue Int. 1982;34:488–494. doi: 10.1007/BF02411290. [DOI] [PubMed] [Google Scholar]
- 11.Murray D W, Rushton N. J Bone Joint Surg. 1990;72:988–992. doi: 10.1302/0301-620X.72B6.2246303. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland K, Mahoney J R, II, Coury A J, Eaton J W. J Clin Invest. 1993;92:2360–2367. doi: 10.1172/JCI116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Q, Topham N S, Anderson J M, Hiltner A, Lodoen G, Payet C R. J Biomed Mater Res. 1990;24:621–637. doi: 10.1002/jbm.820250205. [DOI] [PubMed] [Google Scholar]
- 14.Chan S C, Birdsell D C, Gradeen C Y. Clin Chem. 1991;37:756–758. [PubMed] [Google Scholar]
- 15.Picha G J, Goldstein J A, Store E. Plast Reconstr Surg. 1990;85:903–916. doi: 10.1097/00006534-199006000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Stark G B, Gobel M, Jaeger K. Ann Plast Surg. 1990;24:156–161. doi: 10.1097/00000637-199002000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Behling C A, Spector M. J Biomed Mater Res. 1986;20:653–666. doi: 10.1002/jbm.820200509. [DOI] [PubMed] [Google Scholar]
- 18.Christenson L, Aebischer P, McMillan P, Galletti P M. J Biomed Mater Res. 1989;23:705–718. doi: 10.1002/jbm.820230704. [DOI] [PubMed] [Google Scholar]
- 19.Vistnes L M, Ksander G A, Kosek J. Plast Reconstr Surg. 1978;62:580–588. doi: 10.1097/00006534-197810000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Tang L, Ugarova T P, Plow E F, Eaton J W. J Clin Invest. 1996;97:1329–1334. doi: 10.1172/JCI118549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang L, Eaton J W. In: Tissue Engineering of Vascular Grafts. Zilla P P, Greisler H P, editors. Georgetown, TX: Landes; 1998. , in press. [Google Scholar]
- 22.Guo W, Willen R, Andersson R, Parsson H, Liu X, Johansson K, Bengmark S. Int J Artif Organs. 1993;16:276–284. [PubMed] [Google Scholar]
- 23.Christenson L, Wahlberg L, Aebischer P. J Biomed Mater Res. 1991;25:1119–1131. doi: 10.1002/jbm.820250906. [DOI] [PubMed] [Google Scholar]
- 24.Jones D A, Abbassi O, McIntire L V, McEver R P, Smith C W. Biophys J. 1993;112:749–759. doi: 10.1016/S0006-3495(93)81195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asako H, Kurose I, Wolf R, DeFrees S, Zheng Z-L, Philips M L, Paulson J C, Granger D N. J Clin Invest. 1994;93:1508–1515. doi: 10.1172/JCI117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubes P, Kanwar S. J Immunol. 1994;152:3570–3577. [PubMed] [Google Scholar]
- 27.Kottke-Marchant K, Anderson J M, Umemure Y, Marchant R E. Biomaterials. 1989;10:147–155. doi: 10.1016/0142-9612(89)90017-3. [DOI] [PubMed] [Google Scholar]
- 28.Yates S G, II, Nakagawa Y, Berger K, Sauvage L R. Surg Gynecol Obstet. 1973;136:12–16. [PubMed] [Google Scholar]
- 29.Ward R, Minns R J. Biomaterials. 1989;10:425–428. doi: 10.1016/0142-9612(89)90135-x. [DOI] [PubMed] [Google Scholar]
- 30.Hamlin G W, Rajah S M, Crow M J, Kester R C. Br J Surg. 1978;65:272–276. doi: 10.1002/bjs.1800650416. [DOI] [PubMed] [Google Scholar]
- 31.Doolittle R F, Schubert D, Schwartz S A. Arch Biochim Biophys. 1967;118:456–457. doi: 10.1016/0003-9861(67)90374-8. [DOI] [PubMed] [Google Scholar]
- 32.Beaven M A, Robinson-White A, Roderick N B, Kauffman G L. Klin Wochenschr. 1982;60:873–881. doi: 10.1007/BF01716943. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira S H, Ng K K, Vane J R. Br J Pharmacol. 1973;49:543–553. doi: 10.1111/j.1476-5381.1973.tb17265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irman-Florjanc, T. & Erjavec, F. (1994) Agents Actions 41, special issue, C129–C130. [DOI] [PubMed]
- 35.Atkinson T P, White M V, Kaliner M A. In: Inflammation: Basic Principles and Clinical Correlates. 2nd Ed. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1992. pp. 193–209. [Google Scholar]
- 36.Kaplan A P, Beaven M A. J Invest Dermatol. 1976;67:327–332. doi: 10.1111/1523-1747.ep12514352. [DOI] [PubMed] [Google Scholar]
- 37.Himmelhoch S R, Evans W H, Mage M G, Peterson E A. Biochemistry. 1969;8:914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- 38.Yam L T, Li C Y, Crosby W H. Am J Clin Pathol. 1971;55:283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- 39.Torres J L, Rush R S, Main A R. Arch Biochem Biophys. 1988;267:271–279. doi: 10.1016/0003-9861(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 40.Reiner E, Aldridge W N, Hoskin F C G. Enzyme Hydrolysing Organophosphorous Compounds. New York: Horwood; 1989. p. 266. [Google Scholar]
- 41.Akerstrom G, Lisander B. Acta Anesthesiol Scand. 1994;38:569–574. doi: 10.1111/j.1399-6576.1994.tb03953.x. [DOI] [PubMed] [Google Scholar]
- 42.Gilman A G, Rall T W, Nies A S, Taylor P. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 8th Ed. Oxford: Pergamon; 1990. p. 1811. [Google Scholar]
- 43.Kaneta S, Yanaguimoto H, Kagaya J, Ishizuki S, Fujihira E. Res Commun Chem Pathol Pharmacol. 1993;79:167–184. [PubMed] [Google Scholar]
- 44.Yoshihara S, Chan B, Yamawaki I, Geppetti P, Ricciardolo F L M, Massion P P, Nadel J A. Am J Respir Crit Care Med. 1995;151:1011–1017. doi: 10.1164/ajrccm/151.4.1011. [DOI] [PubMed] [Google Scholar]
- 45.Ginsburg H, Ben-Shahar D, Hammel I, Ben-David E. Nature (London) 1979;280:151–153. doi: 10.1038/280151a0. [DOI] [PubMed] [Google Scholar]
- 46.Boyd J F, Smith A N. J Pathol Bacteriol. 1959;78:379–388. doi: 10.1002/path.1700780205. [DOI] [PubMed] [Google Scholar]
- 47.Kitamura Y, Go S, Hatanaka K. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 48.Chervenick P A, Boggs D R. J Cell Physiol. 1969;73:25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- 49.Ebbe S, Phalen E, Stohlman F., Jr Blood. 1973;42:857–864. [PubMed] [Google Scholar]
- 50.Wershil B K, Murakami T, Galli S J. J Immunol. 1988;140:2356–2360. [PubMed] [Google Scholar]
- 51.Ramos B F, Qureshi R, Olsen K M, Jakschik B A. J Immunol. 1990;145:1868–1873. [PubMed] [Google Scholar]
- 52.Ramos B F, Zhang Y, Qureshi R, Jakschik B A. J Immunol. 1991;147:1636–1641. [PubMed] [Google Scholar]
- 53.Ramos B F, Zhang Y, Angkachatchai V, Jakschik B A. J Pharmacol Exp Ther. 1992;262:559–565. [PubMed] [Google Scholar]
- 54.Wei Y, Heghinian K, Bell R, Jakschik B A. J Immunol. 1986;137:1993–2000. [PubMed] [Google Scholar]
- 55.Zhang Y, Ramos B F, Jakschik B A. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- 56.Fujita J, Nakayama H, Onoue H, Kanakura Y, Nakano T, Asai H, Takeda S-I, Honjo T, Kitamura Y. J Cell Physiol. 1983;134:78–84. doi: 10.1002/jcp.1041340109. [DOI] [PubMed] [Google Scholar]
- 57.Asako H, Kurose I, Wolfe R, DeFrees S, Zheng Z-L, Philips M L, Paulson J C, Granger D N. J Clin Invest. 1994;93:1508–1515. doi: 10.1172/JCI117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubes P, Kanwar S. J Immunol. 1994;152:3570–3577. [PubMed] [Google Scholar]
- 59.Riley J F, West G B. J Physiol. 1953;129:528–537. doi: 10.1113/jphysiol.1953.sp004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto H, Nakano K. Immunology. 1990;69:162–165. [PMC free article] [PubMed] [Google Scholar]
- 61.Zwadlo-Klarwasser G, Braam U, Muhl-Zurbes P, Schmutzler W. Agents Actions. 1994;41:C99–100. doi: 10.1007/BF02007785. [DOI] [PubMed] [Google Scholar]
- 62.Metcalfe D D, Costa J J, Burd P R. In: Inflammation: Basic Principles and Clinical Correlates. 2nd Ed. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1992. pp. 709–725. [Google Scholar]
- 63.Maslinski C. Agents Actions. 1975;5:89–107. doi: 10.1007/BF02027348. [DOI] [PubMed] [Google Scholar]
- 64.White M V, Kaliner M A. In: Inflammation: Basic Principles and Clinical Correlates. 2nd Ed. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1988. pp. 169–187. [Google Scholar]
- 65.Falus A, Meretey K. Immunol Today. 1992;13:154–156. doi: 10.1016/0167-5699(92)90117-p. [DOI] [PubMed] [Google Scholar]
- 66.Mortillaro N A, Granger D N, Kvietys P R, Rutili G, Taylor A E. Am J Physiol. 1981;240:G381–G386. doi: 10.1152/ajpgi.1981.240.5.G381. [DOI] [PubMed] [Google Scholar]
- 67.Issekutz A C. Lab Invest. 1981;45:234–240. [PubMed] [Google Scholar]
- 68.Paterson I S, Klausoner J M, Goldman G, Welbourn R, Alexander J S, Shepro D, Hechtman H B. Microvasc Res. 1989;38:49–56. doi: 10.1016/0026-2862(89)90016-2. [DOI] [PubMed] [Google Scholar]
- 69.Grega G J, Adamski S W. Microcirc Endothel Lymph. 1991;7:267–291. [PubMed] [Google Scholar]
- 70.Geng J M, Bevilacqua M P, Moore K L, McIntyre T M, Prescott S M, Kim J M, Bliss G A, Zimmerman G A, McEver R P. Nature (London) 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 71.Hattori R, Hamilton K K, Fugate R D, McEver B R, Sims P J. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 72.Owen D A A, Woodward D F. Biochem Soc Trans. 1980;8:150–155. doi: 10.1042/bst0080150. [DOI] [PubMed] [Google Scholar]
- 73.Marks R, Greaves M W. Br J Clin Pharmacol. 1977;4:367–369. doi: 10.1111/j.1365-2125.1977.tb00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Owen D A A, Poy E, Woodward D F, Daniel D. Br J Pharmacol. 1980;69:615–623. doi: 10.1111/j.1476-5381.1980.tb07912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toda N. Circ Res. 1987;61:280–286. doi: 10.1161/01.res.61.2.280. [DOI] [PubMed] [Google Scholar]
- 76.Franzen L. Acta Path Microbiol Scand. 1981;89:57–62. doi: 10.1111/j.1699-0463.1981.tb00187.x. [DOI] [PubMed] [Google Scholar]