Abstract
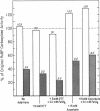
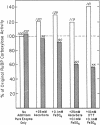
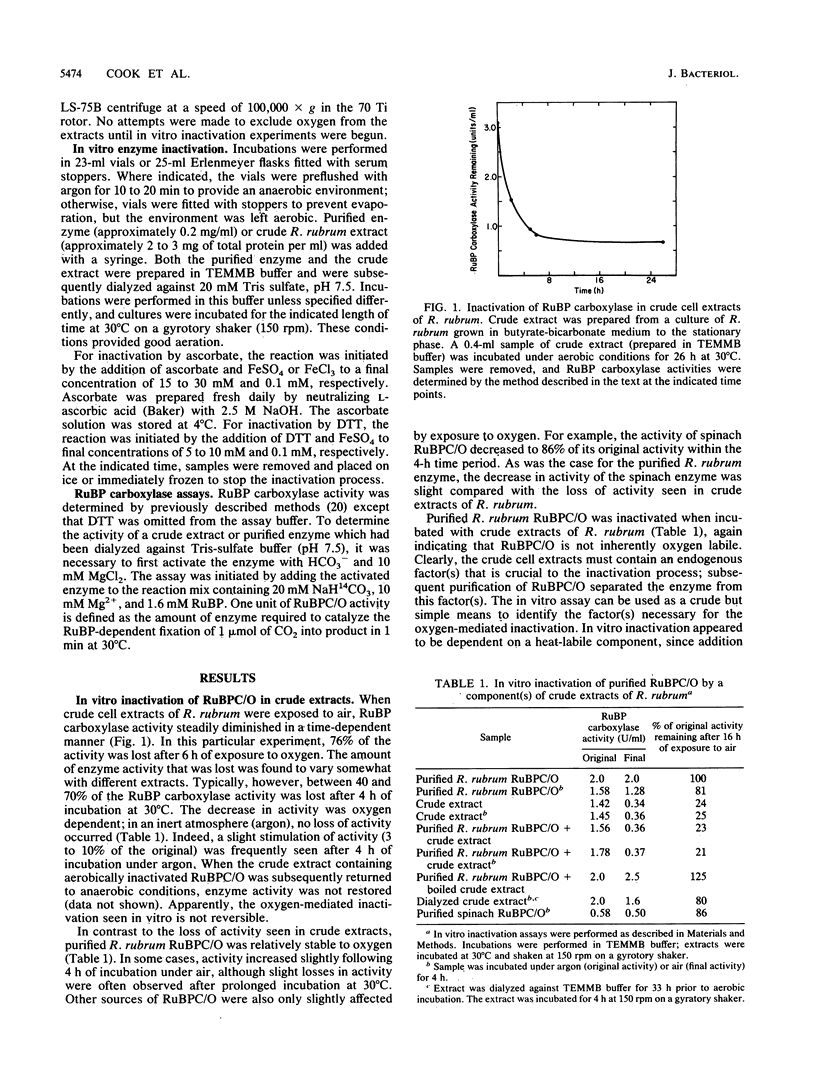
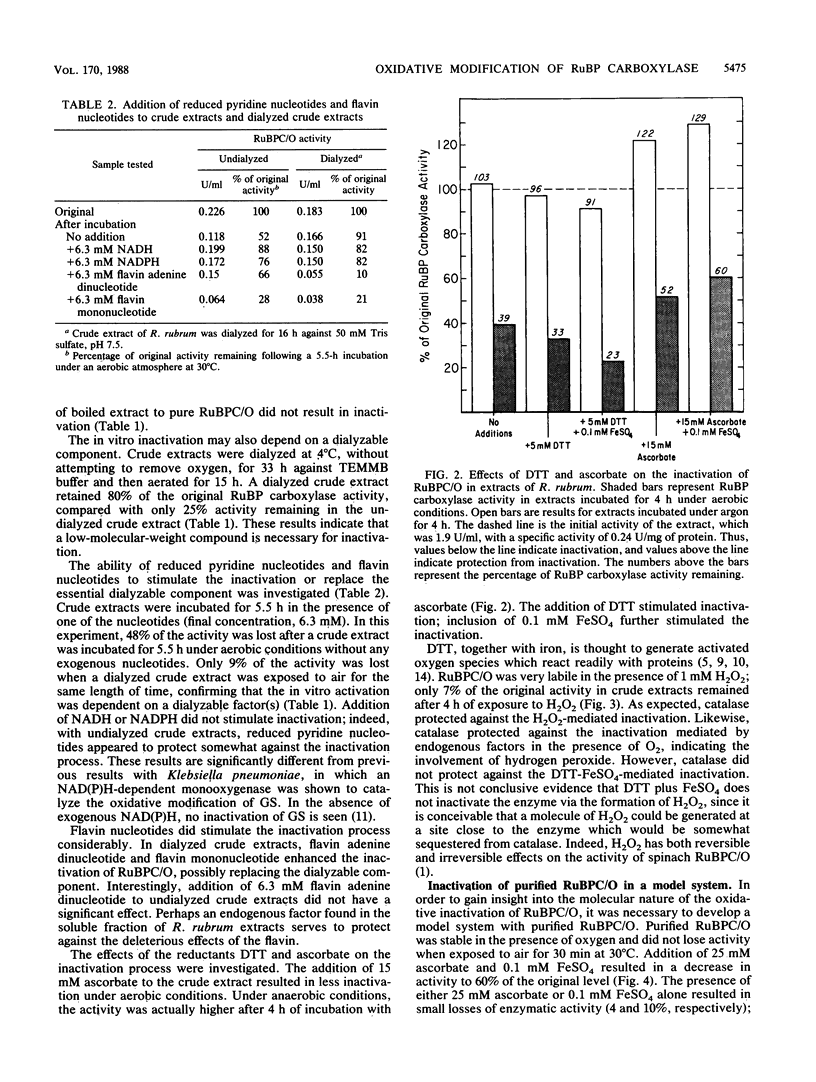
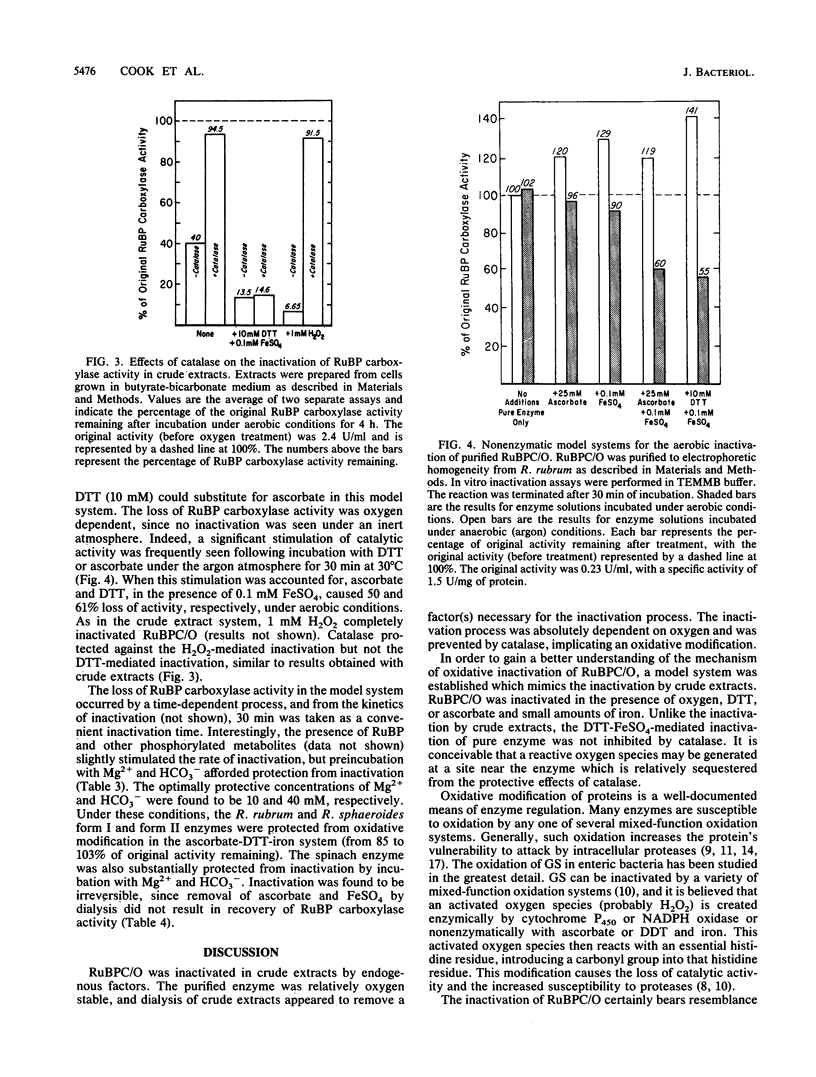
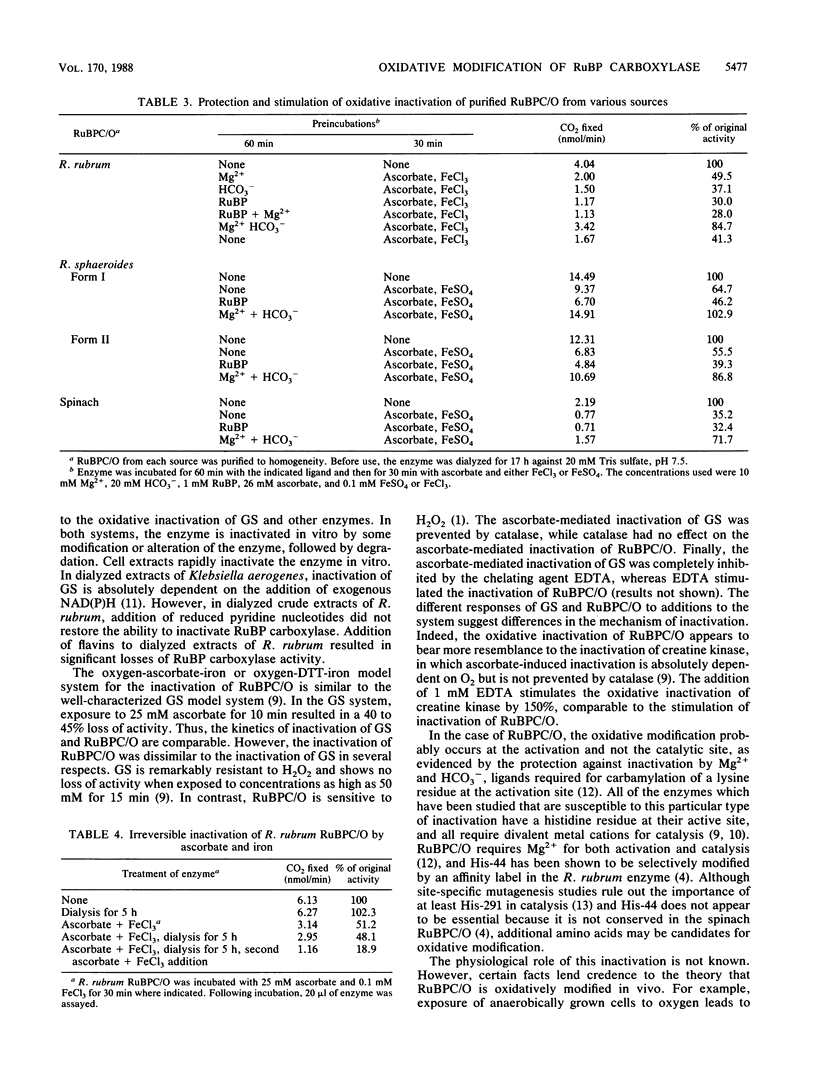
Ribulose 1,5-bisphosphate (RuBP) carboxylase/oxygenase (RuBPC/O) was inactivated in crude extracts of Rhodospirillum rubrum under atmospheric levels of oxygen; no inactivation occurred under an atmosphere of argon. RuBP carboxylase activity did not decrease in dialyzed extracts, indicating that a dialyzable factor was required for inactivation. The inactivation was inhibited by catalase. Purified RuBPC/O is relatively oxygen stable, as no loss of activity was observed after 4 h under an oxygen atmosphere. The aerobic inactivation catalyzed by endogenous factors in crude extracts was mimicked by using a model system containing purified enzyme, ascorbate, and FeSO4 or FeCl3. Dithiothreitol was found to substitute for ascorbate in the model system. Preincubation of the purified enzyme with RuBP led to enhanced inactivation, whereas Mg2+ and HCO3- significantly protected against inactivation. Unlike the inactivation catalyzed by endogenous factors from extracts of R. rubrum, inactivation in the model system was not inhibited by catalase. It is proposed that ascorbate and iron, in the presence of oxygen, generate a reactive oxygen species which reacts with a residue at the activation site, rendering the enzyme inactive.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J., Canvin D. T., Lorimer G. H. Interactions of hydrogen peroxide with ribulose bisphosphate carboxylase oxygenase. J Biol Chem. 1980 Aug 25;255(16):7870–7875. [PubMed] [Google Scholar]
- Cook L. S., Tabita F. R. Oxygen regulation of ribulose 1,5-bisphosphate carboxylase activity in Rhodospirillum rubrum. J Bacteriol. 1988 Dec;170(12):5468–5472. doi: 10.1128/jb.170.12.5468-5472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon C. S., Hartman F. C. 2-(4-Bromoacetamido)anilino-2-deoxypentitol 1,5-bisphosphate, a new affinity label for ribulose bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Determination of reaction parameters and characterization of an active site peptide. J Biol Chem. 1984 Mar 10;259(5):3102–3110. [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Lerch K. Primary structure of tyrosinase from Neurospora crassa. II. Complete amino acid sequence and chemical structure of a tripeptide containing an unusual thioether. J Biol Chem. 1982 Jun 10;257(11):6414–6419. [PubMed] [Google Scholar]
- Levine R. L. Covalent modification of proteins by mixed function oxidation. Curr Top Cell Regul. 1985;27:305–316. doi: 10.1016/b978-0-12-152827-0.50033-5. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J Biol Chem. 1983 Oct 10;258(19):11823–11827. [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. II. Characterization of the ascorbate model system. J Biol Chem. 1983 Oct 10;258(19):11828–11833. [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Niyogi S. K., Foote R. S., Mural R. J., Larimer F. W., Mitra S., Soper T. S., Machanoff R., Hartman F. C. Nonessentiality of histidine 291 of Rhodospirillum rubrum ribulose-bisphosphate carboxylase/oxygenase as determined by site-directed mutagenesis. J Biol Chem. 1986 Aug 5;261(22):10087–10092. [PubMed] [Google Scholar]
- Pierce J., Reddy G. S. The sites for catalysis and activation of ribulosebisphosphate carboxylase share a common domain. Arch Biochem Biophys. 1986 Mar;245(2):483–493. doi: 10.1016/0003-9861(86)90241-9. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. Preferential degradation of the oxidatively modified form of glutamine synthetase by intracellular mammalian proteases. J Biol Chem. 1985 Jan 10;260(1):300–305. [PubMed] [Google Scholar]
- Rivett A. J. The effect of mixed-function oxidation of enzymes on their susceptibility to degradation by a nonlysosomal cysteine proteinase. Arch Biochem Biophys. 1985 Dec;243(2):624–632. doi: 10.1016/0003-9861(85)90540-5. [DOI] [PubMed] [Google Scholar]
- Roseman J. E., Levine R. L. Purification of a protease from Escherichia coli with specificity for oxidized glutamine synthetase. J Biol Chem. 1987 Feb 15;262(5):2101–2110. [PubMed] [Google Scholar]
- Sarles L. S., Tabita F. R. Derepression of the synthesis of D-ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J Bacteriol. 1983 Jan;153(1):458–464. doi: 10.1128/jb.153.1.458-464.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification, and effects of metallic ions. J Biol Chem. 1974 Jun 10;249(11):3453–3458. [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Switzer R. L. Oxygen-dependent inactivation of glutamine phosphoribosylpyrophosphate amidotransferase in stationary-phase cultures of Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):108–114. doi: 10.1128/jb.121.1.108-114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]