Abstract
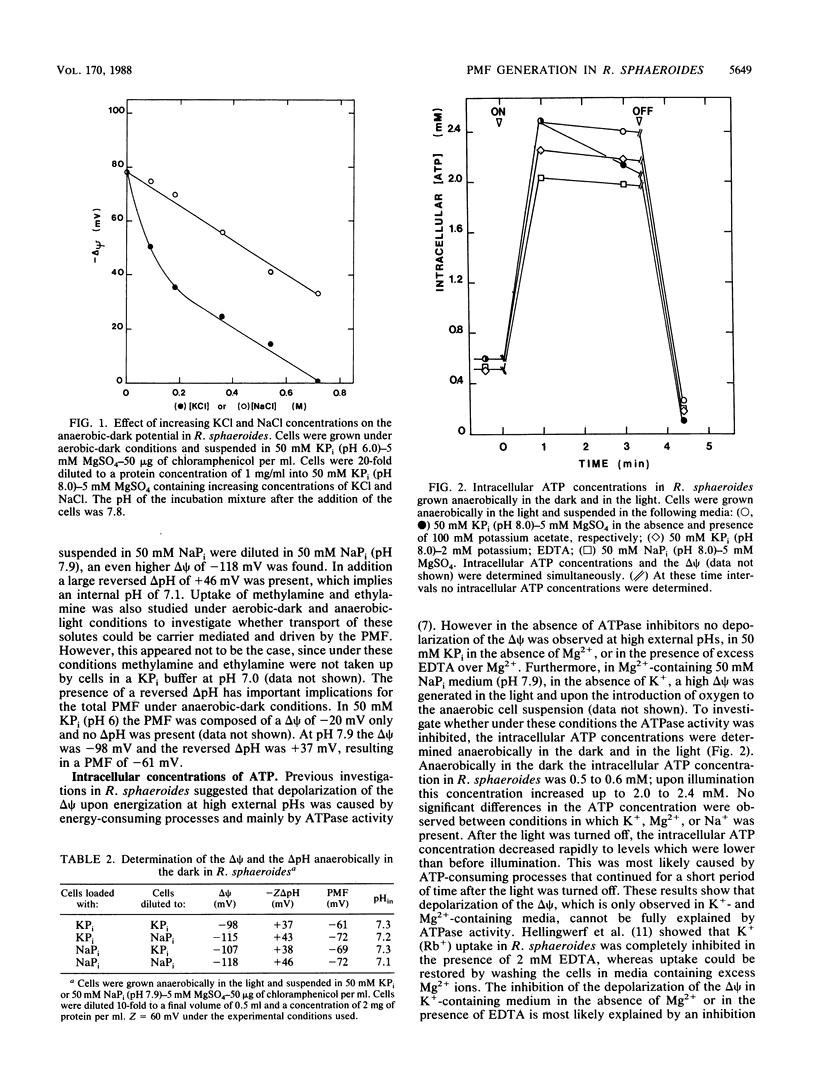
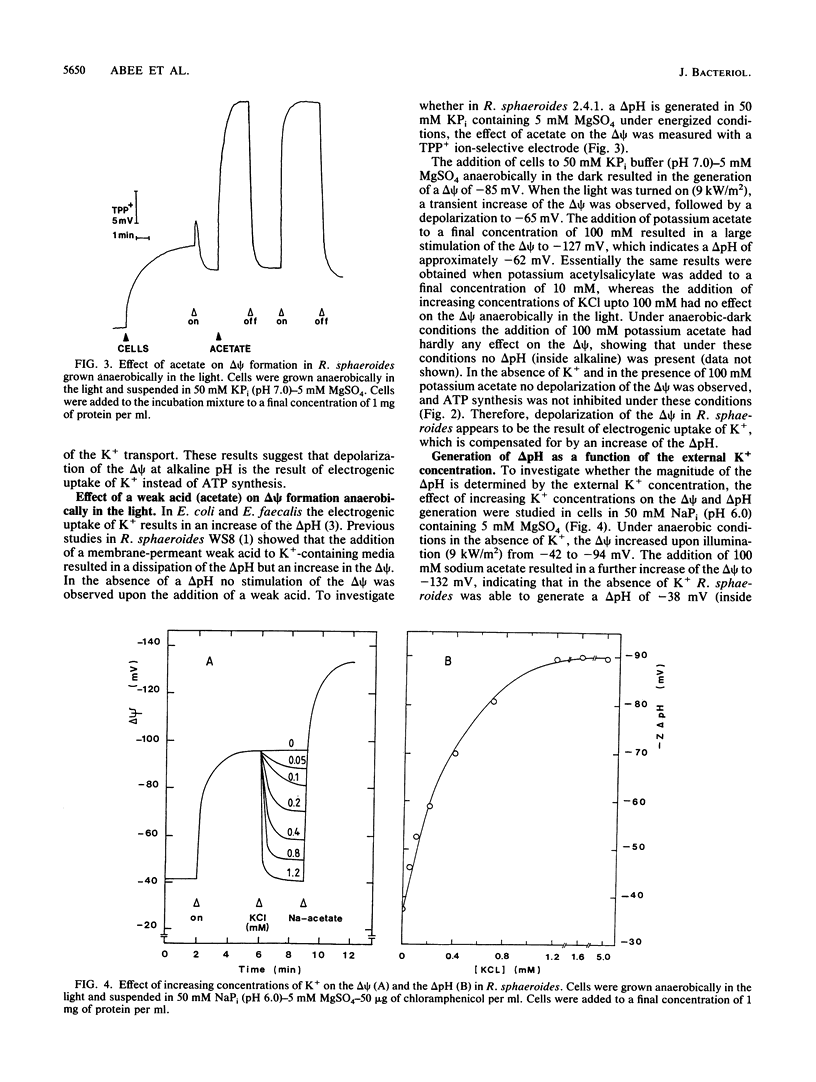
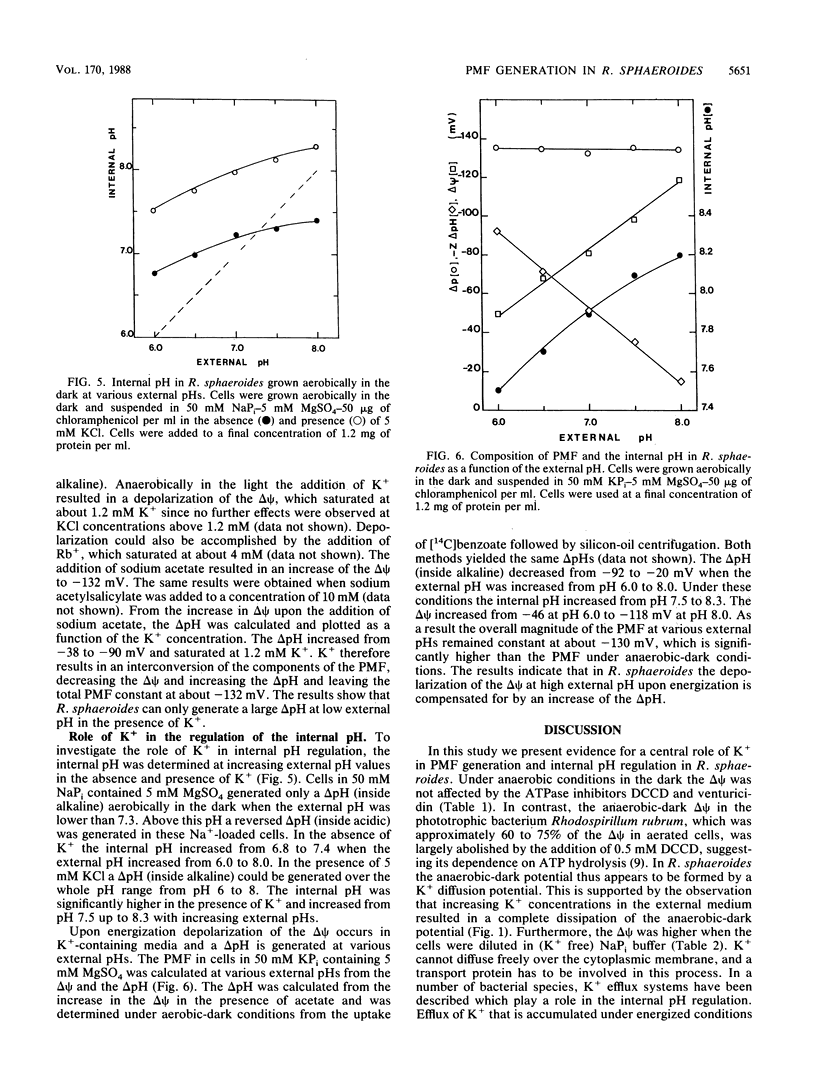
The proton motive force (PMF) was determined in Rhodobacter sphaeroides under anaerobic conditions in the dark and under aerobic-dark and anaerobic-light conditions. Anaerobically in the dark in potassium phosphate buffer, the PMF at pH 6 was -20 mV and was composed of an electrical potential (delta psi) only. At pH 7.9 the PMF was composed of a high delta psi of -98 mV and was partially compensated by a reversed pH gradient (delta pH) of +37 mV. ATPase inhibitors did not affect the delta psi, which was most likely the result of a K+ diffusion potential. Under energized conditions in the presence of K+ the delta psi depolarized due to electrogenic K+ uptake. This led to the generation of a delta pH (inside alkaline) in the external pH range of 6 to 8. This delta pH was dependent on the K+ concentration and was maximal at external K+ concentrations larger than 1.2 mM. In energized cells in 50 mM KPi buffer containing 5 mM MgSO4, a delta pH (inside alkaline) was present at external pHs from pH 6 to 8. As a result the overall magnitude of the PMF at various external pHs remained constant at -130 mV, which was significantly higher than the PMF under anaerobic-dark conditions. In the absence of K+, in 50 mM NaPi buffer containing 5 mM MgSO4, no depolarization of the delta psi was found and the PMF was composed of a large delta psi and a small delta pH. The delta pH became even reversed (inside acidic) at alkaline pHs (pH>7.3), resulting in a lowering of the PMF. These results demonstrate that in R. sphaeroides K+ uptake is essential for the generation of a delta pH and plays a central role in the regulation of the internal pH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Booth I. R., Dinnbier U., Epstein W., Gajewska A. Evidence for multiple K+ export systems in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3743–3749. doi: 10.1128/jb.169.8.3743-3749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R., Mitchell W. J., Hamilton W. A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979 Sep 15;182(3):687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink M. G., Hellingwerf K. J., Konings W. N. The role of the proton motive force and electron flow in solute transport in Escherichia coli. Eur J Biochem. 1985 Nov 15;153(1):161–165. doi: 10.1111/j.1432-1033.1985.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Effects of K+ on the proton motive force of Bradyrhizobium sp. strain 32H1. J Bacteriol. 1986 May;166(2):618–622. doi: 10.1128/jb.166.2.618-622.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellingwerf K. J., Friedberg I., Lolkema J. S., Michels P. A., Konings W. N. Energy coupling of facilitated transport of inorganic ions in Rhodopseudomonas sphaeroides. J Bacteriol. 1982 Jun;150(3):1183–1191. doi: 10.1128/jb.150.3.1183-1191.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper P. Potassium transport system of Rhodopseudomonas capsulata. J Bacteriol. 1978 Mar;133(3):1314–1322. doi: 10.1128/jb.133.3.1314-1322.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Barker S. L. Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J Bacteriol. 1977 Jun;130(3):1017–1023. doi: 10.1128/jb.130.3.1017-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Effects of K+ and Na+ on the proton motive force of respiring Escherichia coli at alkaline pH. J Bacteriol. 1985 Aug;163(2):423–429. doi: 10.1128/jb.163.2.423-429.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll R. G., Booth I. R. The relationship between intracellular pH, the pH gradient and potassium transport in Escherichia coli. Biochem J. 1983 Dec 15;216(3):709–716. doi: 10.1042/bj2160709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll R. G., Booth I. R. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem J. 1981 Sep 15;198(3):691–698. doi: 10.1042/bj1980691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Lolkema J., Hellingwerf K. J., Kaptein R., Konings W. N. Quantitative agreement between the values for the light-induced delta pH in Rhodopseudomonas sphaeroides measured with automated follow-dialysis and 31P NMR. FEBS Lett. 1981 Jan 26;123(2):319–323. doi: 10.1016/0014-5793(81)80318-3. [DOI] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Stewart L. M., Bakker E. P., Booth I. R. Energy coupling to K+ uptake via the Trk system in Escherichia coli: the role of ATP. J Gen Microbiol. 1985 Jan;131(1):77–85. doi: 10.1099/00221287-131-1-77. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]
- ten Brink B., Otto R., Hansen U. P., Konings W. N. Energy recycling by lactate efflux in growing and nongrowing cells of Streptococcus cremoris. J Bacteriol. 1985 Apr;162(1):383–390. doi: 10.1128/jb.162.1.383-390.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



