Abstract
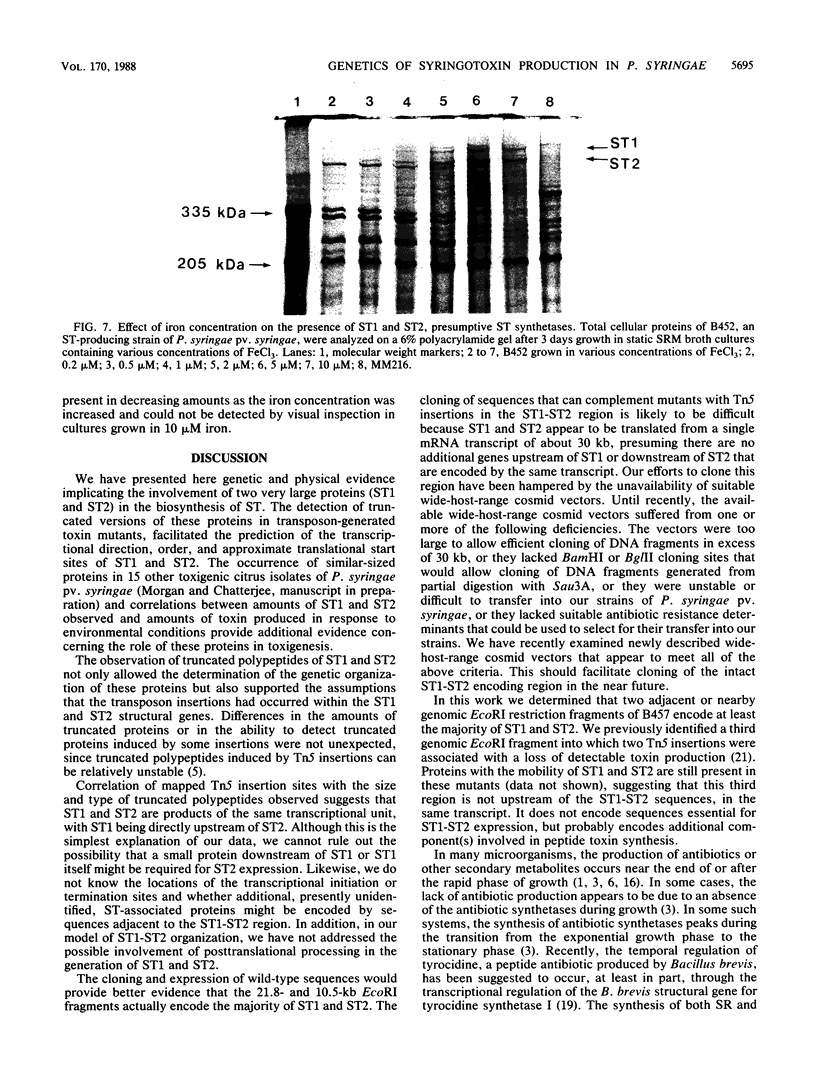
Many strains of Pseudomonas syringae pv. syringae produce one of two low-molecular-weight, peptide-containing phytotoxins, either syringomycin (SR) or syringotoxin (ST). An analysis of Tn5-induced ST-mutants revealed alterations in the presence of two large proteins (ca. 470 and 435 kilodaltons). Apparent truncated forms of the 470 (ST1)- or 435 (ST2)-kilodalton proteins were observed in some mutants. Mapping of the Tn5 insertions and size determinations of truncated proteins suggested that both ST1 and ST2 are in the same transcriptional unit, with ST1 being directly upstream of ST2. When an ST-producing strain of P. syringae pv. syringae was grown under toxin-inducing conditions, ST1 and ST2 were first detected at the end of the exponential phase of growth, which was when the first accumulation of ST was observed. High iron levels were essential for efficient ST production. At concentrations of FeCl3 of between 0.2 and 5 microM, the amount of toxin accumulated was almost directly proportional to the iron concentrations. The amount of ST1 and ST2 present showed a corresponding increase in response to iron concentrations. Our genetic and physiological data implicate ST1 and ST2 in the biosynthesis of syringotoxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y. Nitrogen metabolite regulation of antibiotic biosynthesis. Annu Rev Microbiol. 1980;34:209–233. doi: 10.1146/annurev.mi.34.100180.001233. [DOI] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- Castric P. A. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol. 1975 May;21(5):613–618. doi: 10.1139/m75-088. [DOI] [PubMed] [Google Scholar]
- Giphart-Gassler M., Plasterk R. H., van de Putte P. G inversion in bacteriophage Mu: a novel way of gene splicing. Nature. 1982 May 27;297(5864):339–342. doi: 10.1038/297339a0. [DOI] [PubMed] [Google Scholar]
- Gross D. C. Regulation of syringomycin synthesis in Pseudomonas syringae pv. syringae and defined conditions for its production. J Appl Bacteriol. 1985 Feb;58(2):167–174. doi: 10.1111/j.1365-2672.1985.tb01444.x. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Merrick M. J. Genetics of antibiotic production. Bacteriol Rev. 1977 Sep;41(3):595–635. doi: 10.1128/br.41.3.595-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Zuber P., Czekay G., Losick R. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. K., Chatterjee A. K. Isolation and characterization of Tn5 insertion mutants of Pseudomonas syringae pv. syringae altered in the production of the peptide phytotoxin syringotoxin. J Bacteriol. 1985 Oct;164(1):14–18. doi: 10.1128/jb.164.1.14-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967 Nov;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. W., Gross D. C. Evaluation of the Role of Syringomycin in Plant Pathogenesis by Using Tn5 Mutants of Pseudomonas syringae pv. syringae Defective in Syringomycin Production. Appl Environ Microbiol. 1988 Jun;54(6):1345–1353. doi: 10.1128/aem.54.6.1345-1353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. W., Gross D. C. Physical and functional analyses of the syrA and syrB genes involved in syringomycin production by Pseudomonas syringae pv. syringae. J Bacteriol. 1988 Dec;170(12):5680–5688. doi: 10.1128/jb.170.12.5680-5688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]






