Abstract
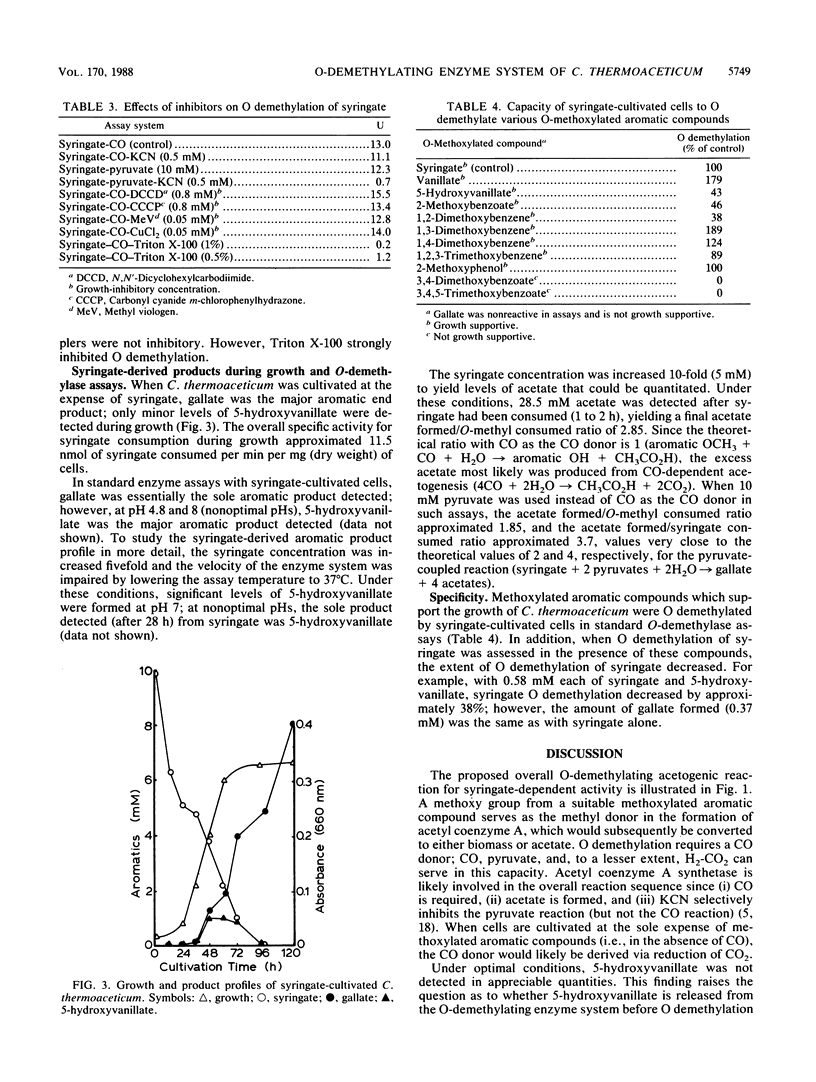
An inducible O-demethylating enzyme system was characterized from Clostridium thermoaceticum cultivated at the expense of syringate. Glucose and methanol, but not CO, partially repressed its expression. Induced whole cells catalyzed the carbon monoxide (CO)-dependent O demethylation of methoxylated aromatic compounds with the concomitant formation of acetate. Pyruvate and, to a lesser extent, H2-CO2 could replace CO in these reactions. KCN inhibited pyruvate-dependent activity but not the CO-dependent activity. The ATPase inhibitor N,N'-dicyclohexylcarbodiimide, the protonophore carbonyl cyanide m-chlorophenylhydrazone, and methyl viologen did not appreciably inhibit O demethylation by induced cells, whereas Triton X-100 was inhibitory. The enzyme system appeared to convert syringate sequentially to 5-hydroxyvanillate and gallate. The proposed overall reaction stoichiometry was as follows: syringate + 2CO + 2H2O----gallate + 2 acetates. Growth-supportive methoxylated aromatic compounds were O demethylated by syringate-cultivated cells and inhibitory to syringate O demethylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryson M. F., Drake H. L. Energy-dependent transport of nickel by Clostridium pasteurianum. J Bacteriol. 1988 Jan;170(1):234–238. doi: 10.1128/jb.170.1.234-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeerd K. A., Saxena A., Nagle D. P., Jr, Suflita J. M. Metabolism of the 18O-methoxy substituent of 3-methoxybenzoic acid and other unlabeled methoxybenzoic acids by anaerobic bacteria. Appl Environ Microbiol. 1988 May;54(5):1237–1242. doi: 10.1128/aem.54.5.1237-1242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J Biol Chem. 1980 Aug 10;255(15):7174–7180. [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Young L. Y. A gram-negative anaerobic bacterium that utilizes o-methyl substituents of aromatic acids. Appl Environ Microbiol. 1985 May;49(5):1345–1347. doi: 10.1128/aem.49.5.1345-1347.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Young L. Y. Anaerobic c(1) metabolism of the o-methyl-C-labeled substituent of vanillate. Appl Environ Microbiol. 1986 Jan;51(1):84–87. doi: 10.1128/aem.51.1.84-87.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Drake H. L. Development of a minimally defined medium for the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Aug;159(2):700–703. doi: 10.1128/jb.159.2.700-703.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Savage M. D., Drake H. L. Adaptation of the acetogen Clostridium thermoautotrophicum to minimal medium. J Bacteriol. 1986 Jan;165(1):315–318. doi: 10.1128/jb.165.1.315-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharak Genthner B. R., Bryant M. P. Additional characteristics of one-carbon-compound utilization by Eubacterium limosum and Acetobacterium woodii. Appl Environ Microbiol. 1987 Mar;53(3):471–476. doi: 10.1128/aem.53.3.471-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]