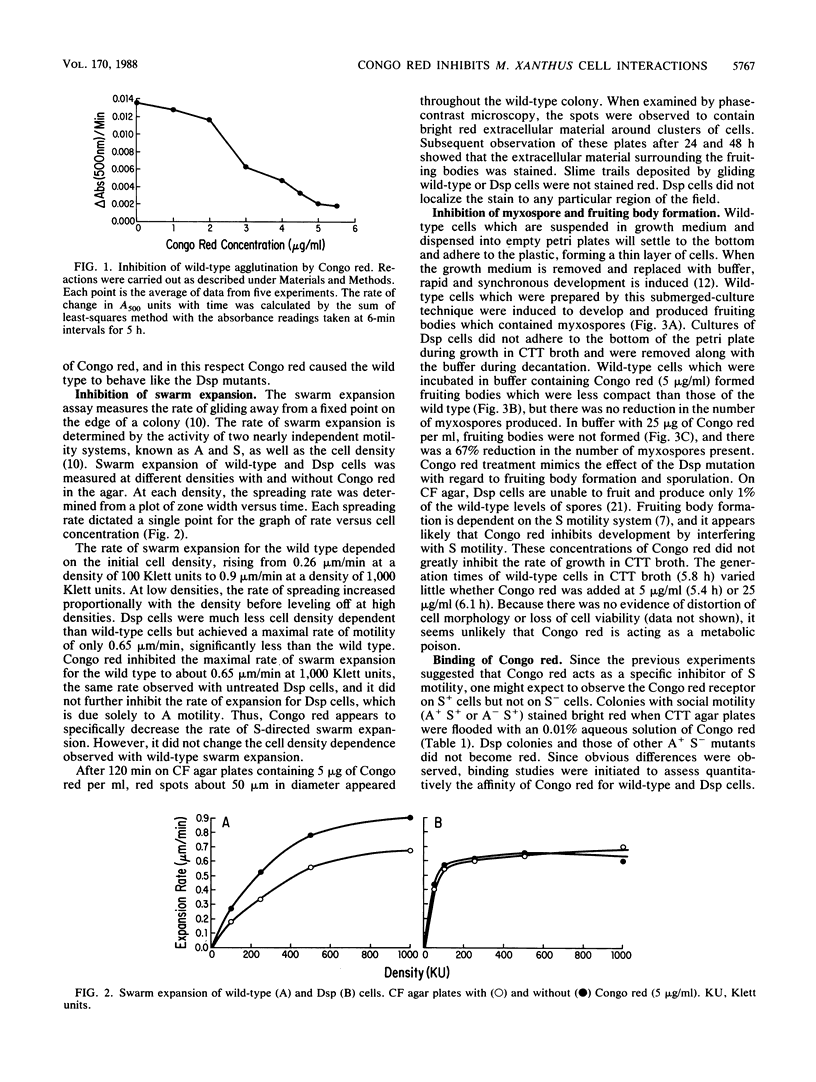
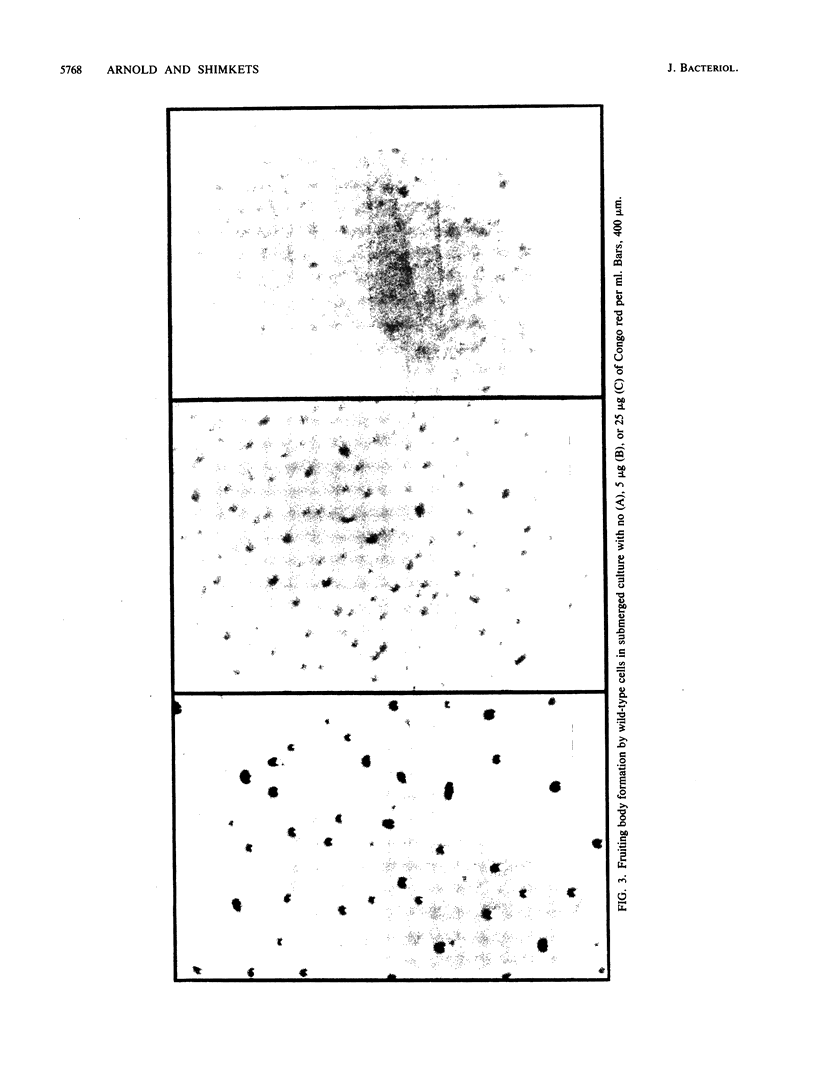
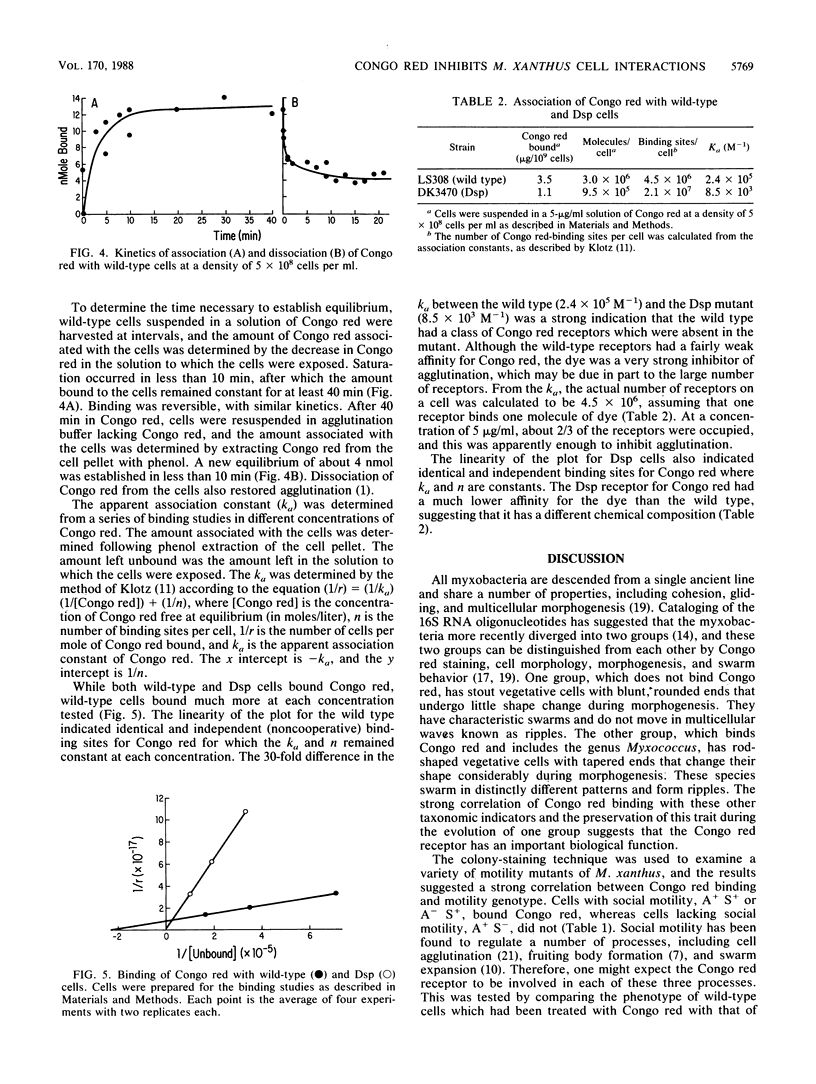
Abstract
The function of molecules associated with the cell surface may be determined by examining the phenotype of cells treated with inhibitors specific to these cell surface molecules. This strategy was used to examine the function of the major Congo red receptor of the myxobacterium Myxococcus xanthus, which has a developmental cycle that involves social interactions among cells. A class of social motility mutations (A+ S-), known as dsp, may inhibit the same subcellular component as Congo red because the phenotype of wild-type cells which had been treated with Congo red resembled in several ways the phenotype of the Dsp mutants. First, Congo red inhibited agglutination of wild-type cells, whereas Dsp cells were incapable of agglutinating, even in the absence of Congo red. Second, Congo red inhibited fruiting body formation by wild-type cells and reduced the yield of myxospores. Untreated Dsp cells were unable to form fruiting bodies and produced few myxospores. Third, Congo red reduced the rate of wild-type gliding motility to a level comparable to that of untreated Dsp cells, but did not inhibit the A motility of Dsp cells. Finally, binding studies showed that Dsp cells lacked the major Congo red receptor. Wild-type cells bound Congo red with an apparent association constant of 2.4 X 10(5) M-1, while Dsp cells bound it with an apparent association constant of 8.5 X 10(3) M-1. Binding of Congo red to wild-type cells was saturated in less than 10 min and was reversible when excess Congo red was removed. These results suggest that the Congo red receptors are controlled by the S motility system and that these receptors are involved in cell cohesion, social motility, and fruiting body formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J. W., Shimkets L. J. Cell surface properties correlated with cohesion in Myxococcus xanthus. J Bacteriol. 1988 Dec;170(12):5771–5777. doi: 10.1128/jb.170.12.5771-5777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnosspelius G. Myxobacterial slime and proteolytic activity. Arch Microbiol. 1978 Jan 23;116(1):51–59. doi: 10.1007/BF00408733. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Chilton W. S. Galactosamine glycan of Chondrococcus columnaris. Science. 1966 May 27;152(3726):1247–1248. doi: 10.1126/science.152.3726.1247. [DOI] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982 Jul;151(1):458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Sodergren E., Masuda T., Kaiser D. Systematic isolation of transducing phages for Myxococcus xanthus. Virology. 1978 Jul 1;88(1):44–53. doi: 10.1016/0042-6822(78)90108-3. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983 May;154(2):906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy H. D. Studies on the taxonomy of the Myxobacterales. I. Record of Canadian isolates and survey of methods. Can J Microbiol. 1969 Dec;15(12):1453–1461. doi: 10.1139/m69-259. [DOI] [PubMed] [Google Scholar]
- Orndorff P., Stellwag E., Starich T., Dworkin M., Zissler J. Genetic and physical characterization of lysogeny by bacteriophage MX8 in Myxococcus xanthus. J Bacteriol. 1983 May;154(2):772–779. doi: 10.1128/jb.154.2.772-779.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986 Jun;166(3):837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982 Oct;152(1):451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986 Jun;166(3):842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Ultrasonication-an enrichment technique for microcyst-forming bacteria. J Appl Bacteriol. 1976 Aug;41(1):185–188. doi: 10.1111/j.1365-2672.1976.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Karlinsey J. E., Marks J. R., Hurlbert R. E. Identification of a new virulence locus in Agrobacterium tumefaciens that affects polysaccharide composition and plant cell attachment. J Bacteriol. 1987 Jul;169(7):3209–3216. doi: 10.1128/jb.169.7.3209-3216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]




