Abstract
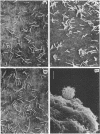
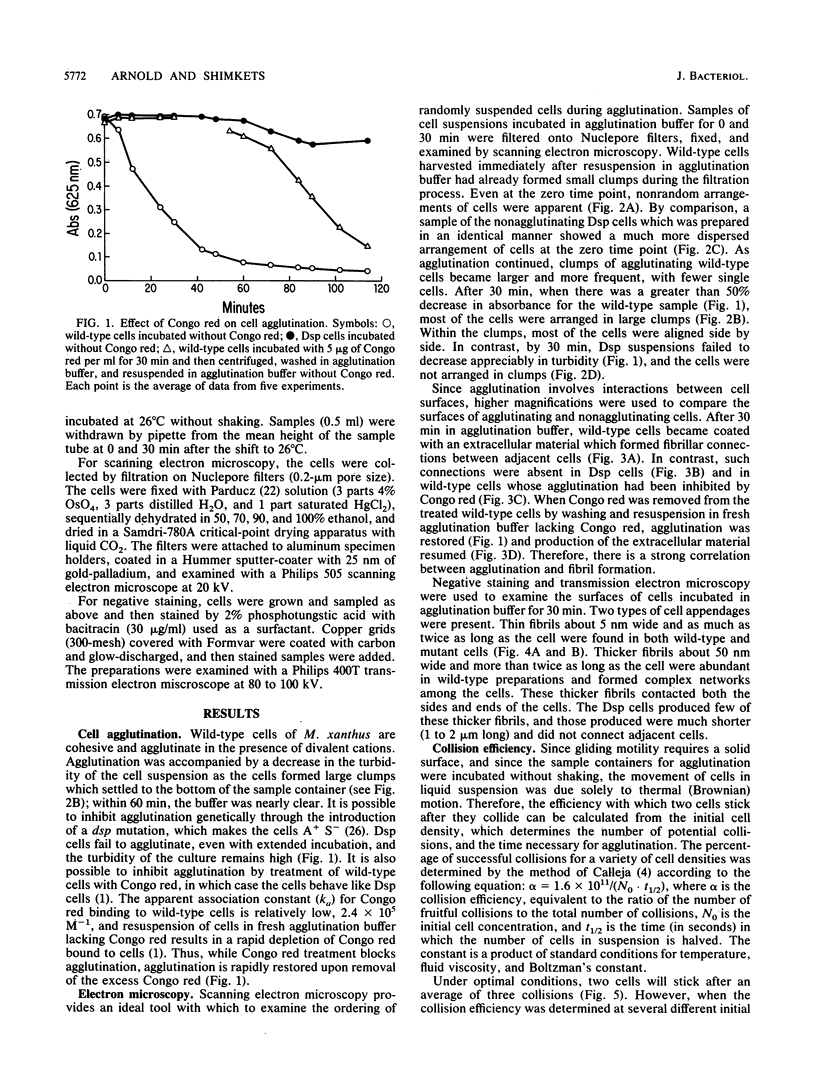
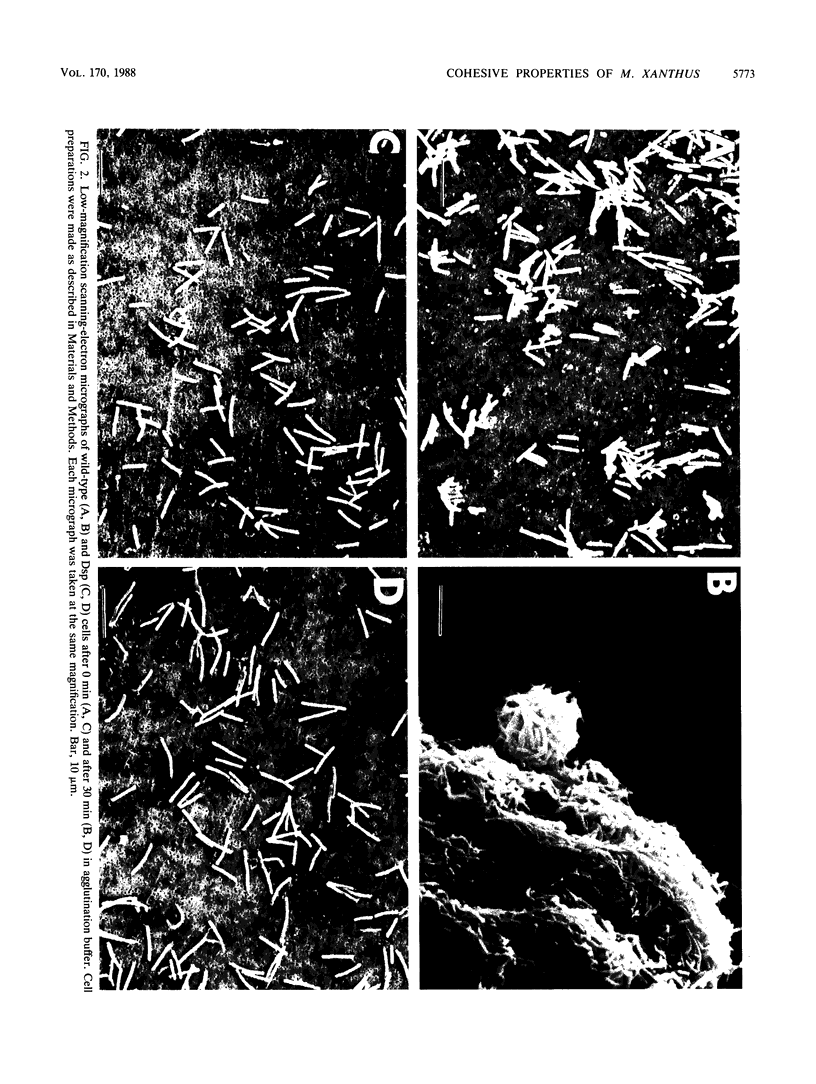
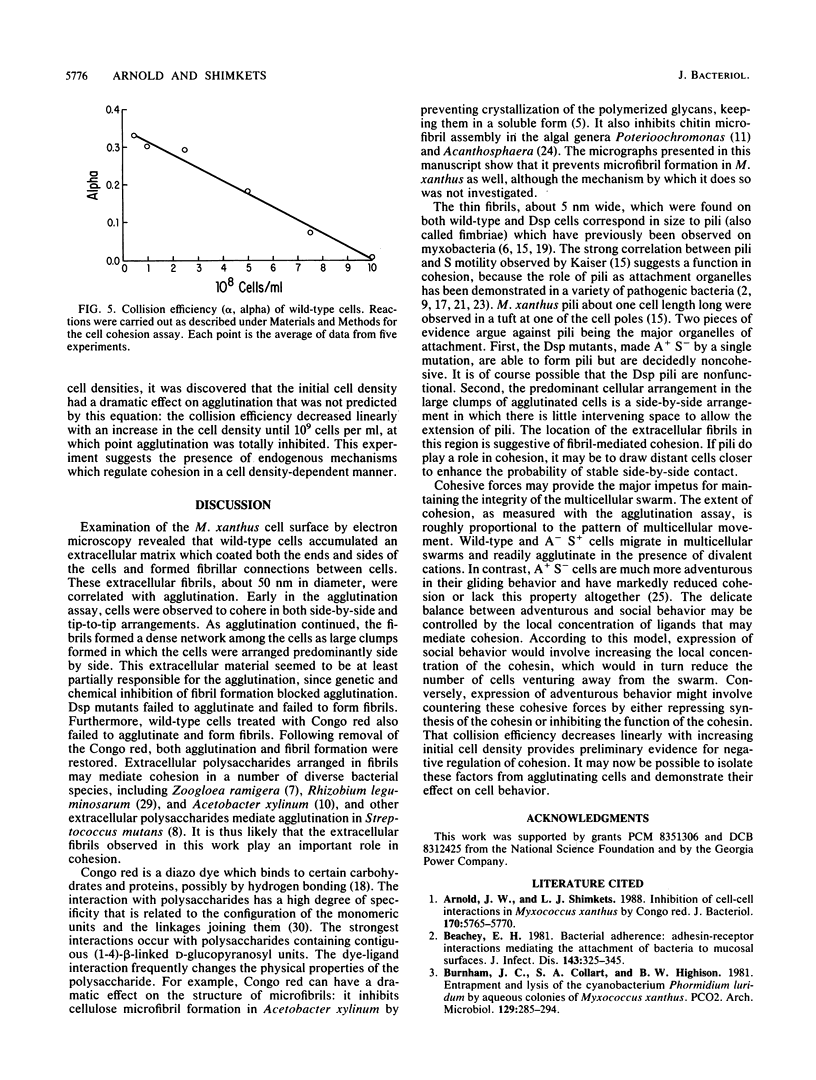
The gliding behavior of Myxococcus xanthus cells is controlled by two multigene systems, A and S, which encode information for adventurous and social behaviors, respectively. The S system can be genetically disrupted through mutation, such as a dsp mutation, or phenotypically disrupted by treating cells with the diazo dye Congo red (Arnold and Shimkets, J. Bacteriol. 170:5765-5770, 1988). One of the functions controlled by the S system is cell agglutination. Immediately after the induction of agglutination, wild-type cells begin to form aggregates, and within 30 min the cells are packed side-to-side in clumps containing thousands of cells. Changes in the cohesive properties of S+ cells are correlated with changes in the topology of the cell surface observed by electron microscopy. Two types of cell-associated appendages were observed on wild-type cells: thin filaments (ca. 5 nm in diameter), which have been called fimbriae or pili, at one cell pole, and thick, flaccid filaments (ca. 50 nm in diameter), referred to as fibrils, at both the sides and tips of cells. Cohesion was correlated with the secretion of the thick fibrils, which coat the cell surface and form an extracellular matrix in which the cells are interconnected. Several lines of evidence suggest that these thick fibrils are involved in cohesion. First, Dsp cells were unable to agglutinate or secrete this extracellular material. Second, wild-type cells which were treated with Congo red neither agglutinated nor secreted the extracellular fibrils. Finally, removal of the Congo red from wild-type cells restored cohesion and also restored production of the thick fibrils. Attempts to estimate the efficiency with which two cells cohered following collision suggested that under optimal conditions, one in three collisions resulted in stable contact. The collision efficiency decreased linearly as the cell density increased, suggesting a cell density-dependent regulation of cohesion. Some aspects of gliding behavior can be explained in terms of an inducer and an inhibitor of S motility.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J. W., Shimkets L. J. Inhibition of cell-cell interactions in Myxococcus xanthus by congo red. J Bacteriol. 1988 Dec;170(12):5765–5770. doi: 10.1128/jb.170.12.5765-5770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Easson D. D., Jr, Sinskey A. J., Peoples O. P. Isolation of Zoogloea ramigera I-16-M exopolysaccharide biosynthetic genes and evidence for instability within this region. J Bacteriol. 1987 Oct;169(10):4518–4524. doi: 10.1128/jb.169.10.4518-4524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Schoolnik G. K., Kessler T. W., Goldmann D. A. Purification and characterization of Haemophilus influenzae pili, and their structural and serological relatedness to Escherichia coli P and mannose-sensitive pili. J Exp Med. 1985 Jan 1;161(1):145–159. doi: 10.1084/jem.161.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler C. H., Brown R. M., Jr, Benziman M. Calcofluor white ST Alters the in vivo assembly of cellulose microfibrils. Science. 1980 Nov 21;210(4472):903–906. doi: 10.1126/science.7434003. [DOI] [PubMed] [Google Scholar]
- Herth W. Calcofluor white and Congo red inhibit chitin microfibril assembly of Poterioochromonas: evidence for a gap between polymerization and microfibril formation. J Cell Biol. 1980 Nov;87(2 Pt 1):442–450. doi: 10.1083/jcb.87.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Sellwood R., Shipley P., Dougan G. Genetic analysis of K88-mediated adhesion of enterotoxigenic Escherichia coli. Nature. 1981 May 14;291(5811):122–126. doi: 10.1038/291122a0. [DOI] [PubMed] [Google Scholar]
- MacRae T. H., Dobson W. J., McCurdy H. D. Fimbriation in gliding bacteria. Can J Microbiol. 1977 Aug;23(8):1096–1108. doi: 10.1139/m77-165. [DOI] [PubMed] [Google Scholar]
- McCurdy H. D. Studies on the taxonomy of the Myxobacterales. I. Record of Canadian isolates and survey of methods. Can J Microbiol. 1969 Dec;15(12):1453–1461. doi: 10.1139/m69-259. [DOI] [PubMed] [Google Scholar]
- Párducz B. Ciliary movement and coordination in ciliates. Int Rev Cytol. 1967;21:91–128. doi: 10.1016/s0074-7696(08)60812-8. [DOI] [PubMed] [Google Scholar]
- Robertson J. N., Vincent P., Ward M. E. The preparation and properties of gonococcal pili. J Gen Microbiol. 1977 Sep;102(1):169–177. doi: 10.1099/00221287-102-1-169. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J. Control of morphogenesis in myxobacteria. Crit Rev Microbiol. 1987;14(3):195–227. doi: 10.3109/10408418709104439. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986 Jun;166(3):837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982 Oct;152(1):451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986 Jun;166(3):842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1987 Sep;169(9):4294–4301. doi: 10.1128/jb.169.9.4294-4301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]