Abstract
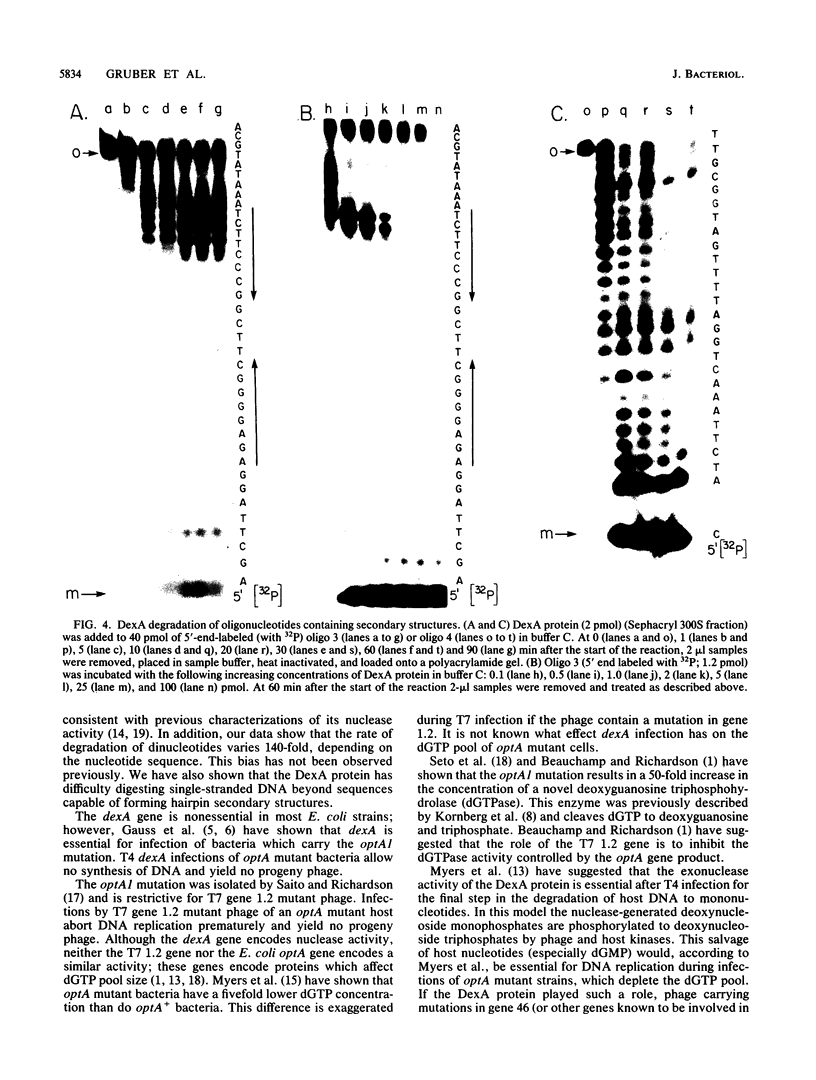
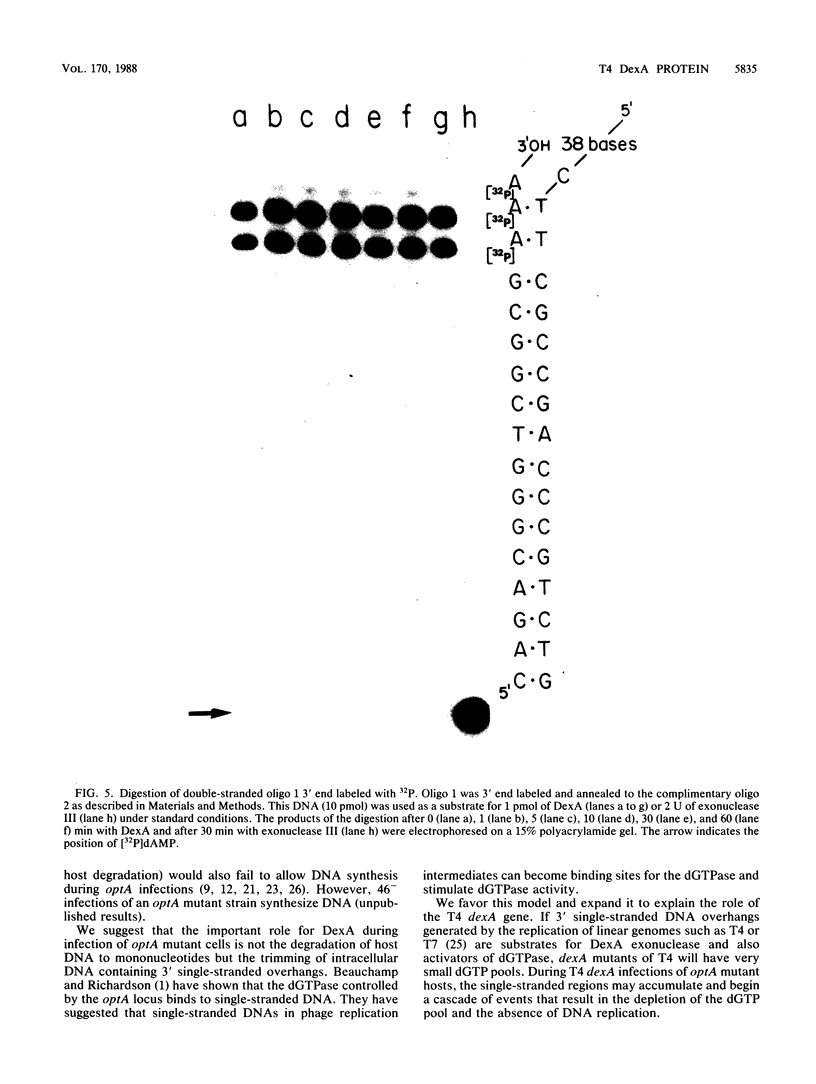
The bacteriophage T4 dexA gene product is required during infection of Escherichia coli strains carrying a mutation in the optA gene. We purified the DexA protein from cells which overproduced the protein. The protein was assayed for nuclease activity on synthetic di- and oligonucleotide substrates of known sequence and secondary structure. Sequence and structure significantly affected nuclease activity. The properties of the enzyme may explain the requirement for the DexA protein during infection of optA mutant hosts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp B. B., Richardson C. C. A unique deoxyguanosine triphosphatase is responsible for the optA1 phenotype of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2563–2567. doi: 10.1073/pnas.85.8.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOLOGY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1607–1620. doi: 10.1073/pnas.45.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Cozzarelli N. R. Genetics and physiology of bacteriophage T4 3'-phosphatase: evidence for involvement of the enzyme in T4 DNA metabolism. J Virol. 1974 Apr;13(4):888–897. doi: 10.1128/jvi.13.4.888-897.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss P., Doherty D. H., Gold L. Bacterial and phage mutations that reveal helix-unwinding activities required for bacteriophage T4 DNA replication. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1669–1673. doi: 10.1073/pnas.80.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss P., Gayle M., Winter R. B., Gold L. The bacteriophage T4 dexA gene: sequence and analysis of a gene conditionally required for DNA replication. Mol Gen Genet. 1987 Jan;206(1):24–34. doi: 10.1007/BF00326532. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Weil J. Deletion analysis of two nonessential regions of the T4 genome. Virology. 1974 Oct;61(2):505–523. doi: 10.1016/0042-6822(74)90286-4. [DOI] [PubMed] [Google Scholar]
- KORNBERG S. R., LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic cleavage of deoxyguanosine triphosphate to deoxyguanosine and tripolyphosphate. J Biol Chem. 1958 Jul;233(1):159–162. [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Little J. W. Mutants of bacteriophage T4 which allow amber mutants of gene 32 to grow in ochre-suppressing hosts. Virology. 1973 May;53(1):47–59. doi: 10.1016/0042-6822(73)90464-9. [DOI] [PubMed] [Google Scholar]
- Mickelson C., Wiberg J. S. Membrane-associated DNase activity controlled by genes 46 and 47 of bacteriophage T4D and elevated DNase activity associated with the T4 das mutation. J Virol. 1981 Oct;40(1):65–77. doi: 10.1128/jvi.40.1.65-77.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. A., Beauchamp B. B., Richardson C. C. Gene 1.2 protein of bacteriophage T7. Effect on deoxyribonucleotide pools. J Biol Chem. 1987 Apr 15;262(11):5288–5292. [PubMed] [Google Scholar]
- OLESON A. E., KOERNER J. F. A DEOXYRIBONUCLEASE INDUCED BY INFECTION WITH BACTERIOPHAGE T2. J Biol Chem. 1964 Sep;239:2935–2943. [PubMed] [Google Scholar]
- Parma D. H., Ingraham L. J., Snyder M. Tandem duplications of the rII region of bacteriophage T4D. Genetics. 1972 Jul;71(3):319–335. doi: 10.1093/genetics/71.3.319. [DOI] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Genetic analysis of gene 1.2 of bacteriophage T7: isolation of a mutant of Escherichia coli unable to support the growth of T7 gene 1.2 mutants. J Virol. 1981 Jan;37(1):343–351. doi: 10.1128/jvi.37.1.343-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short E. C., Jr, Koerner J. F. Separation and characterization of deoxyribonucleases of Escherichia coli B. II. Further purification and properties of an exonuclease induced by infection with bacteriophage T2. J Biol Chem. 1969 Mar 25;244(6):1487–1496. [PubMed] [Google Scholar]
- Singer B. S., Gold L. A mutation that confers temperature sensitivity on the translation of rIIB in bacteriophage T4. J Mol Biol. 1976 May 25;103(3):627–646. doi: 10.1016/0022-2836(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Tuerk C., Gauss P., Thermes C., Groebe D. R., Gayle M., Guild N., Stormo G., d'Aubenton-Carafa Y., Uhlenbeck O. C., Tinoco I., Jr CUUCGG hairpins: extraordinarily stable RNA secondary structures associated with various biochemical processes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1364–1368. doi: 10.1073/pnas.85.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad D. P., Koerner J. F., Childs J. D. Identification and genetic characterization of mutants of bacteriophage T4 defective in the ability to induce exonuclease A. J Virol. 1972 Mar;9(3):399–407. doi: 10.1128/jvi.9.3.399-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H. Function of the bacteriophage T4 transfer RNA's. J Mol Biol. 1973 Mar 15;74(4):753–757. doi: 10.1016/0022-2836(73)90065-x. [DOI] [PubMed] [Google Scholar]