Abstract
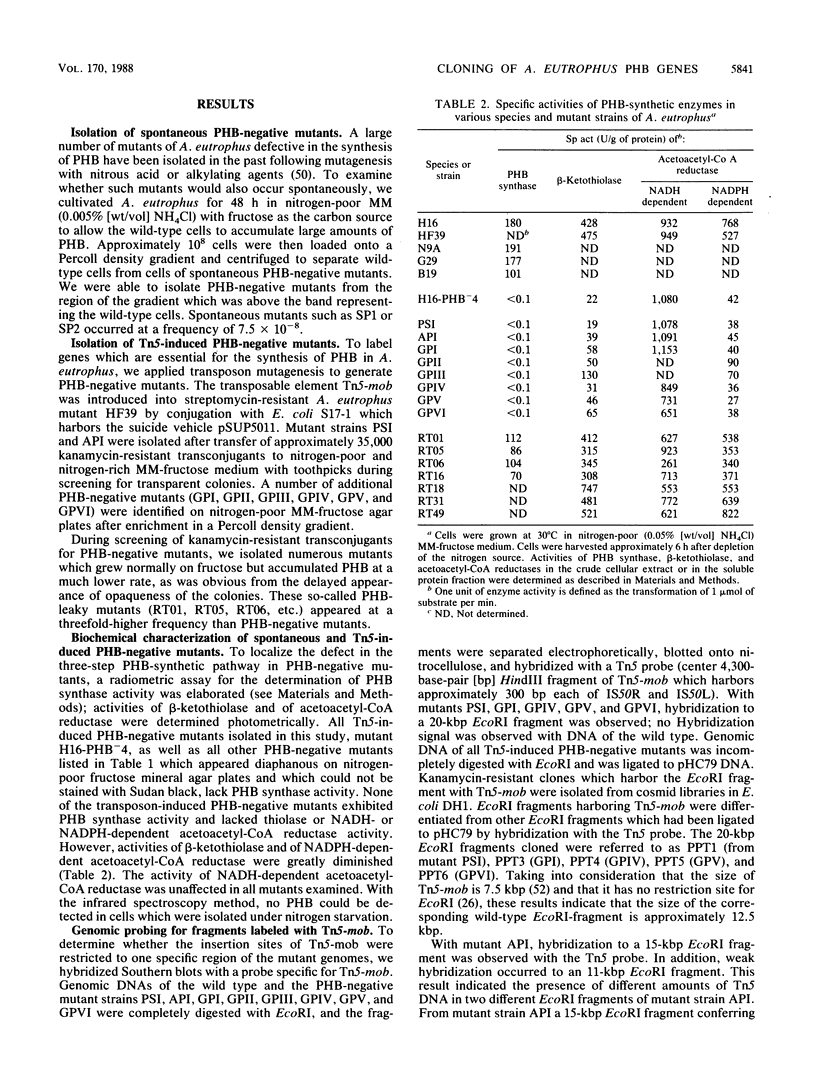
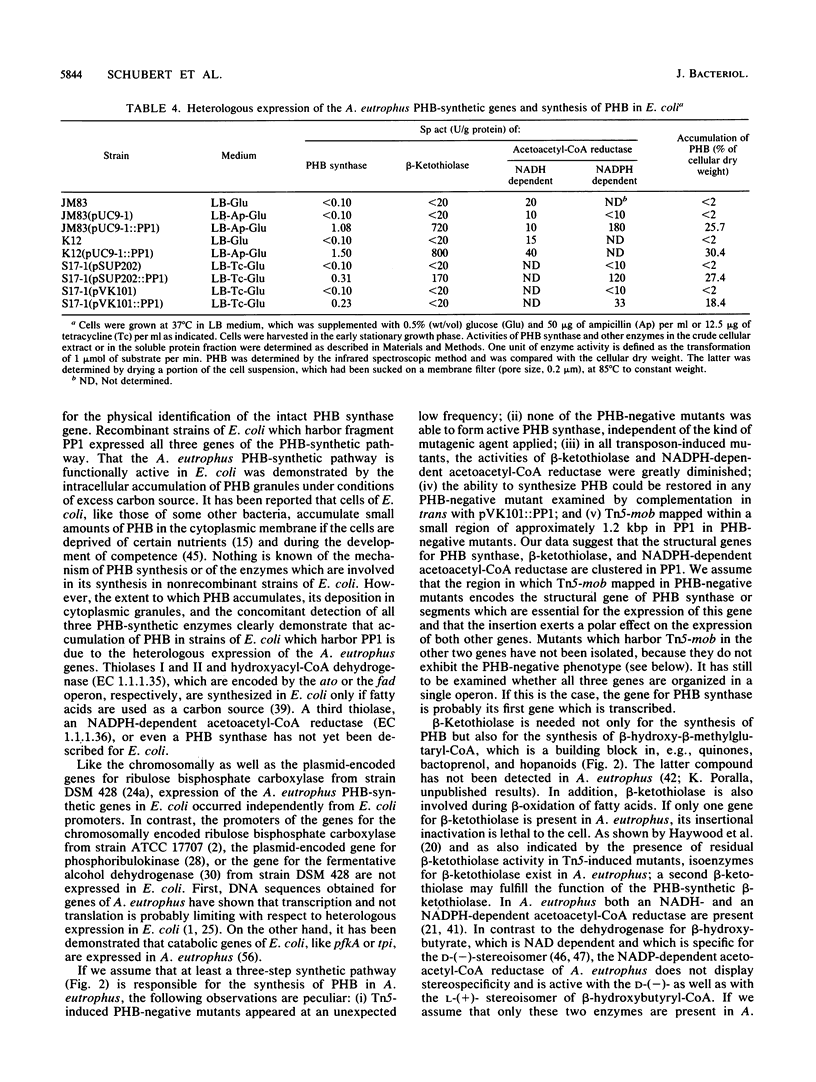
Eight mutants of Alcaligenes eutrophus defective in the intracellular accumulation of poly-beta-hydroxybutyric acid (PHB) were isolated after transposon Tn5 mutagenesis with the suicide vector pSUP5011. EcoRI fragments which harbor Tn5-mob were isolated from pHC79 cosmid gene banks. One of them, PPT1, was used as a probe to detect the intact 12.5-kilobase-pair EcoRI fragment PP1 in a lambda L47 gene bank of A. eutrophus genomic DNA. In six of these mutants (PSI, API, GPI, GPIV, GPV, and GPVI) the insertion of Tn5-mob was physically mapped within a region of approximately 1.2 kilobase pairs in PP1; in mutant API, cointegration of vector DNA has occurred. In two other mutants (GPII and GPIII), most probably only the insertion element had inserted into PP1. All PHB-negative mutants were completely impaired in the formation of active PHB synthase, which was measured by a radiometric assay. In addition, activities of beta-ketothiolase and of NADPH-dependent acetoacetyl coenzyme A (acetoacetyl-CoA) reductase were diminished, whereas the activity of NADPH-dependent acetoacetyl-CoA reductase was unaffected. In all PHB-negative mutants the ability to accumulate PHB was restored upon complementation in trans with PP1. The PHB-synthetic pathway of A. eutrophus was heterologously expressed in Escherichia coli. Recombinant strains of E. coli JM83 and K-12, which harbor pUC9-1::PP1, pSUP202::PP1, or pVK101::PP1, accumulated PHB up to 30% of the cellular dry weight. Crude extracts of these cells had significant activities of the enzymes PHB synthase, beta-ketothiolase, and NADPH-dependent acetoacetyl-CoA reductase. Therefore, PP1 most probably encodes all three genes of the PHB-synthetic pathway in A. eutrophus. In addition to PHB-negative mutants, we isolated mutants which accumulate PHB at a much lower rate than the wild type does. These PHB-leaky mutants exhibited activities of all three PHB-synthetic enzymes; Tn5-mob had not inserted into PP1, and the phenotype of the wild type could not be restored with fragment PP1. The rationale for this mutant type remains unknown.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K., Caton J. Sequence analysis of the Alcaligenes eutrophus chromosomally encoded ribulose bisphosphate carboxylase large and small subunit genes and their gene products. J Bacteriol. 1987 Oct;169(10):4547–4558. doi: 10.1128/jb.169.10.4547-4558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K., Wilke-Douglas M. Genetic and physical mapping and expression in Pseudomonas aeruginosa of the chromosomally encoded ribulose bisphosphate carboxylase genes of Alcaligenes eutrophus. J Bacteriol. 1987 May;169(5):1997–2004. doi: 10.1128/jb.169.5.1997-2004.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Davis J. T., Moore R. N., Imperiali B., Pratt A. J., Kobayashi K., Masamune S., Sinskey A. J., Walsh C. T., Fukui T., Tomita K. Biosynthetic thiolase from zoogloea ramigera. I. Preliminary characterization and analysis of proton transfer reaction. J Biol Chem. 1987 Jan 5;262(1):82–89. [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Hogrefe C., Schlegel H. G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981 Jul;147(1):198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T., Yoshimoto A., Matsumoto M., Hosokawa S., Saito T. Enzymatic synthesis of poly-beta-hydroxybutyrate in Zoogloea ramigera. Arch Microbiol. 1976 Nov 2;110(23):149–156. doi: 10.1007/BF00690222. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK G. DIE BIOSYNTHESE DER POLY-BETA-HYDROXYBUTTERSAEURE DURCH KNALLGASBAKTERIEN. II. VERWERTUNG ORGANISCHER SAEUREN. Arch Mikrobiol. 1964 Feb 21;47:230–235. [PubMed] [Google Scholar]
- Gilbert P., Brown M. R. Effect of R-plasmid RP1 and nutrient depletion on the gross cellular composition of Escherichia coli and its resistance to some uncoupling phenols. J Bacteriol. 1978 Mar;133(3):1062–1065. doi: 10.1128/jb.133.3.1062-1065.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel R. J., Merrick J. M. Metabolism of poly- -hydroxybutyrate: effect of mild alkaline extraction on native poly- -hydroxybutyrate granules. J Bacteriol. 1971 Nov;108(2):782–789. doi: 10.1128/jb.108.2.782-789.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Jendrossek D., Steinbüchel A., Schlegel H. G. Alcohol dehydrogenase gene from Alcaligenes eutrophus: subcloning, heterologous expression in Escherichia coli, sequencing, and location of Tn5 insertions. J Bacteriol. 1988 Nov;170(11):5248–5256. doi: 10.1128/jb.170.11.5248-5256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Jendrossek D., Fründ C., Steinbüchel A., Schlegel H. G. Cloning of the Alcaligenes eutrophus alcohol dehydrogenase gene. J Bacteriol. 1988 Feb;170(2):685–692. doi: 10.1128/jb.170.2.685-692.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- MERRICK J. M., DOUDOROFF M. Enzymatic synthesis of poly-beta-hydroxybutyric acid in bacteria. Nature. 1961 Mar 18;189:890–892. doi: 10.1038/189890a0. [DOI] [PubMed] [Google Scholar]
- Moskowitz G. J., Merrick J. M. Metabolism of poly-beta-hydroxybutyrate. II. Enzymatic synthesis of D-(-)-beta hydroxybutyryl coenzyme A by an enoyl hydrase from Rhodospirillum rubrum. Biochemistry. 1969 Jul;8(7):2748–2755. doi: 10.1021/bi00835a009. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Saito T., Tomita K. Purification and properties of beta-ketothiolase from Zoogloea ramigera. Arch Microbiol. 1978 Jan 23;116(1):21–27. doi: 10.1007/BF00408729. [DOI] [PubMed] [Google Scholar]
- Nunn W. D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986 Jun;50(2):179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeding V., Schlegel H. G. Beta-ketothiolase from Hydrogenomonas eutropha H16 and its significance in the regulation of poly-beta-hydroxybutyrate metabolism. Biochem J. 1973 May;134(1):239–248. doi: 10.1042/bj1340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourisson G., Rohmer M., Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol. 1987;41:301–333. doi: 10.1146/annurev.mi.41.100187.001505. [DOI] [PubMed] [Google Scholar]
- Ploux O., Masamune S., Walsh C. T. The NADPH-linked acetoacetyl-CoA reductase from Zoogloea ramigera. Characterization and mechanistic studies of the cloned enzyme over-produced in Escherichia coli. Eur J Biochem. 1988 May 16;174(1):177–182. doi: 10.1111/j.1432-1033.1988.tb14079.x. [DOI] [PubMed] [Google Scholar]
- Reusch R. N., Hiske T. W., Sadoff H. L. Poly-beta-hydroxybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J Bacteriol. 1986 Nov;168(2):553–562. doi: 10.1128/jb.168.2.553-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER J. DIE SYNTHESE VON POLY-BETA-HYDROXYBUTTERSAEURE DURCH HYDROGENOMONAS H-16 DIE ZU BETA-HYDROXYBUTYRYL-COENZYM A FUEHRENDEN REAKTIONSSCHRITTE. Arch Mikrobiol. 1964 Oct 2;49:236–255. [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schlegel H. G., Lafferty R., Krauss I. The isolation of mutants not accumulating poly-beta-hydroxybutyric acid. Arch Mikrobiol. 1970;71(3):283–294. doi: 10.1007/BF00410161. [DOI] [PubMed] [Google Scholar]
- Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196(3):413–420. doi: 10.1007/BF00436188. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Urban M., Friedrich B. Mutagenesis of Alcaligenes eutrophus by insertion of the drug-resistance transposon Tn5. Arch Microbiol. 1982 May;131(3):203–207. doi: 10.1007/BF00405879. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A. Expression of the Escherichia coli pfkA gene in Alcaligenes eutrophus and in other gram-negative bacteria. J Bacteriol. 1986 Apr;166(1):319–327. doi: 10.1128/jb.166.1.319-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]



