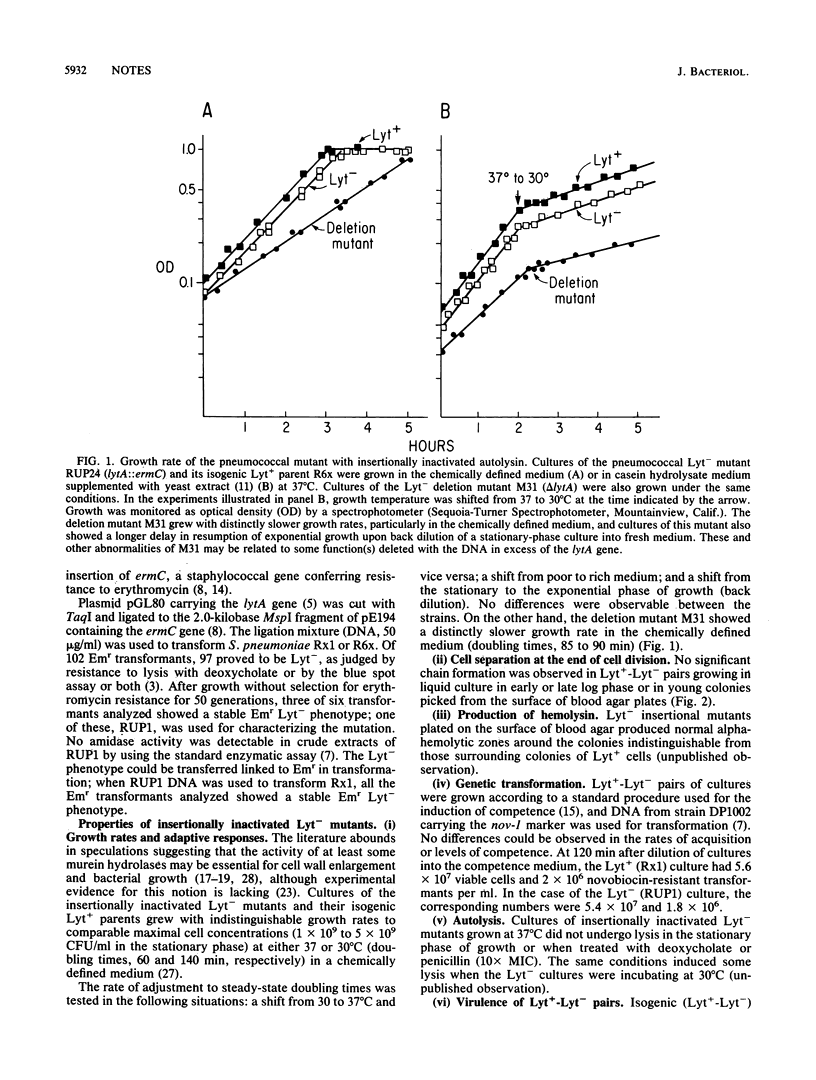
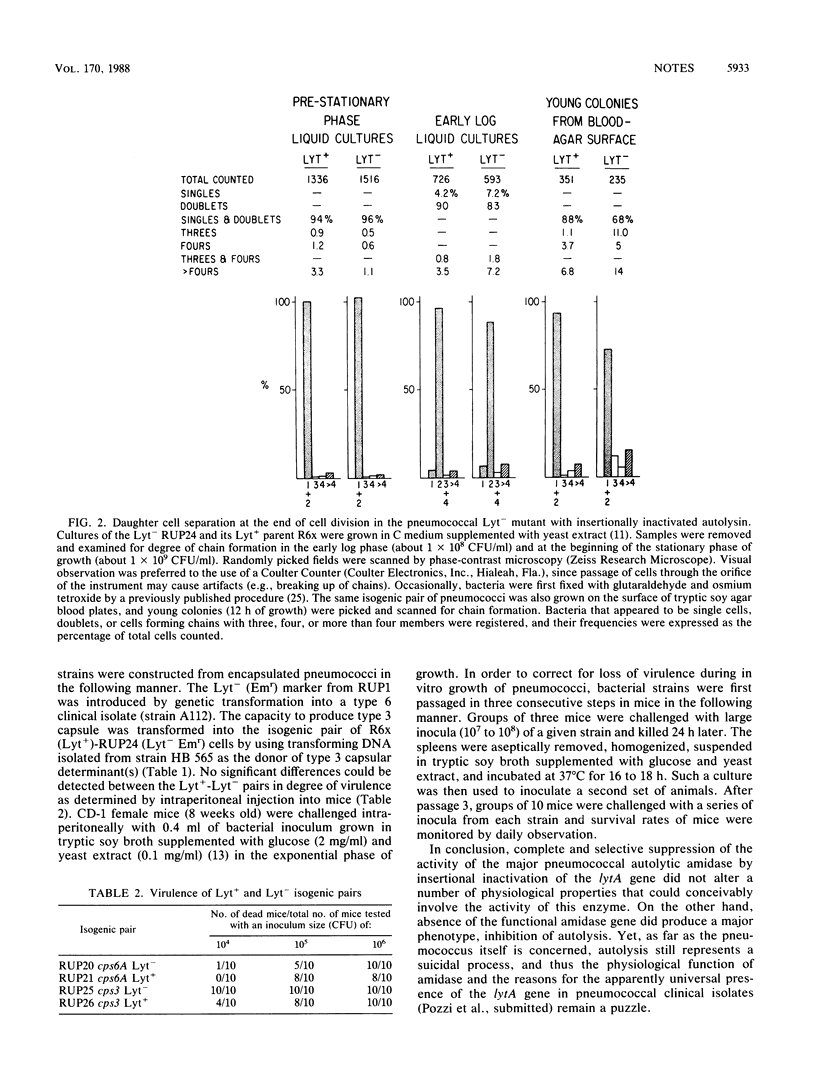
Abstract
The lytA gene encoding the major pneumococcal autolysin (N-acetylmuramoyl-L-alanine amidase) was inactivated by inserting the 2-kilobase MspI fragment of pE194 containing the staphylococcal ermC gene. Stable autolysis-deficient (Lyt-) mutants and their isogenic Lyt+ parents were used in experiments designed to test possible physiological functions of the amidase. No autolysis could be induced in the mutants grown at 37 degrees C by deoxycholate, by incubation in stationary phase, or by treatment with penicillin. On the other hand, the Lyt- mutants exhibited normal growth rates and yields and normal adaptive responses during shifts from one growth temperature or nutritional condition to another. There was no evidence for impeded cell separation (chain formation). Colonies of Lyt- insertional mutants produced normal hemolytic zones on blood agar; they showed normal (high) levels of competence for genetic transformation. Lyt- mutants were also able to produce type 3 and 6 capsular polysaccharides, and such strains showed the same degree of virulence in mice as did the isogenic Lyt+ parent. The physiological function(s) of the amidase remains a puzzle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- García P., García E., Ronda C., Lopez R., Jiang R. Z., Tomasz A. Mutants of Streptococcus pneumoniae that contain a temperature-sensitive autolysin. J Gen Microbiol. 1986 May;132(5):1401–1405. doi: 10.1099/00221287-132-5-1401. [DOI] [PubMed] [Google Scholar]
- García P., García J. L., García E., López R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene. 1986;43(3):265–272. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- Guild W. R., Shoemaker N. B. Mismatch correction in pneumococcal transformation: donor length and hex-dependent marker efficiency. J Bacteriol. 1976 Jan;125(1):125–135. doi: 10.1128/jb.125.1.125-135.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L. V., Gooder H. Specificity of the autolysin of Streptococcus (Diplococcus) pneumoniae. J Bacteriol. 1974 Feb;117(2):796–804. doi: 10.1128/jb.117.2.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Purification of the pneumococcal N-acetylmuramyl-L-alanine amidase to biochemical homogeneity. J Biol Chem. 1976 Jul 25;251(14):4199–4207. [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G., Guild W. R. Modes of integration of heterologous plasmid DNA into the chromosome of Streptococcus pneumoniae. J Bacteriol. 1985 Mar;161(3):909–912. doi: 10.1128/jb.161.3.909-912.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G., Stellini M., Marri L., Molina A. M. Transformation as a tool for studying the epidemiology of tet determinants in Streptococcus pneumoniae. Eur J Epidemiol. 1986 Jun;2(2):90–94. doi: 10.1007/BF00157016. [DOI] [PubMed] [Google Scholar]
- Sanchez-Puelles J. M., Ronda C., Garcia J. L., Garcia P., Lopez R., Garcia E. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur J Biochem. 1986 Jul 15;158(2):289–293. doi: 10.1111/j.1432-1033.1986.tb09749.x. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Suzuki H., Nishimura Y., Mizoguchi J., Hirota Y. On the process of cellular division in Escherichia coli: isolation and characterization of penicillin-binding proteins 1a, 1b, and 3. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4499–4503. doi: 10.1073/pnas.77.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby J. G., Fox M. S. Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3541–3545. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Zanati E., Ziegler R. DNA uptake during genetic transformation and the growing zone of the cell envelope. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1848–1852. doi: 10.1073/pnas.68.8.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]



