Abstract
Background
A randomised controlled study was undertaken to examine the effect of nasal continuous positive airway pressure (CPAP) on 24 hour systemic blood pressure (BP) in patients with obstructive sleep apnoea (OSA).
Methods
Patients were fitted with an ambulatory BP measuring device as outpatients during normal activities and recorded for 24 hours before starting therapeutic or subtherapeutic (4 cm H2O) CPAP treatment. BP monitoring was repeated before completion of 12 weeks of treatment. The primary end point was the change in 24 hour mean BP.
Results
Twenty three of 28 participants in each treatment arm completed the study. There was no significant difference between the two groups in age, body mass index, Epworth Sleepiness Score, apnoea‐hypopnoea index, arousal index, and minimum Sao2. Twenty four patients were hypertensive. The pressure in the therapeutic CPAP group was 10.7 (0.4) cm H2O. CPAP usage was 5.1 (0.4) and 2.6 (0.4) hours/night for the therapeutic and subtherapeutic CPAP groups, respectively (p<0.001). After 12 weeks of treatment there were significant differences between the two CPAP groups in mean (SE) changes in 24 hour diastolic BP (−2.4 (1.2) v 1.1 (1.0) mm Hg (95% CI −6.6 to −0.5), p = 0.025); 24 hour mean BP (−2.5 (1.3) v 1.3 (1.1) mm Hg (95% CI −7.2 to −0.2), p = 0.037); sleep time systolic BP (−4.1 (2.1) v 2.2 (1.8) mm Hg (95% CI −11.8 to −0.7), p = 0.028); and sleep time mean BP (−3.6 (1.7) v 1.3 (1.4) mm Hg (95% CI −9.2 to −0.4), p = 0.033).
Conclusions
Compared with subtherapeutic CPAP, 12 weeks of treatment with therapeutic CPAP leads to reductions in 24 hour mean and diastolic BP by 3.8 mm Hg and 3.5 mm Hg, respectively, in mildly sleepy patients with OSA.
Keywords: blood pressure, continuous positive airway pressure, obstructive sleep apnoea
Obstructive sleep apnoea (OSA) is characterised by repetitive episodes of partial or complete upper airway obstruction causing sleep fragmentation, sleepiness and other symptoms, cognitive function impairment, and poor health status.1 OSA syndrome is equally common among the middle aged white and Hong Kong Chinese populations with a prevalence of at least 4%.2,3,4 Several epidemiological studies have shown an independent association between sleep disordered breathing (SDB) and hypertension.5,6,7 A case‐control study has shown that patients with OSA have increased ambulatory diastolic blood pressure (BP) both day and night, with increased systolic BP at night.8 The Sleep Heart Health Study has shown that SDB was relatively more strongly associated with self‐reported stroke and heart failure than with coronary artery disease.9 A 7 year longitudinal study of otherwise healthy patients with OSA has shown a higher incidence (56.8%) of at least one cardiovascular complication in patients incompletely treated than in those effectively treated, who have a low incidence (6.7%) similar to normal controls.10 Untreated OSA is associated with increased risks of developing non‐fatal and fatal cardiovascular events11 including sudden death.12 There is recent evidence that OSA increases the risk of both myocardial infarction and stroke,13 and nasal CPAP may protect against death from cardiovascular diseases.14
Nasal CPAP is the gold standard treatment for symptomatic patients with OSA, with robust evidence demonstrating improvement of symptoms, cognitive function, and health status in patients treated with CPAP.15,16 Although hypertension is probably a major mechanism linking OSA and cardiovascular complications, there are conflicting data as to whether nasal CPAP can effectively reduce BP in patients with OSA.17,18,19,20,21,22,23,24 This study aimed to assess the medium term effects of nasal CPAP on 24 hour BP in patients newly diagnosed with OSA.
Methods
Subjects
A prospective, randomised, subtherapeutic CPAP controlled parallel study was conducted on 100 consecutive patients newly diagnosed with OSA, as defined by overnight polysomnography (PSG) showing an apnoea‐hypopnoea index (AHI) of ⩾5/hour of sleep plus excessive daytime sleepiness or two of the following symptoms: choking or gasping during sleep, recurrent awakenings from sleep, unrefreshed sleep, daytime fatigue, and impaired concentration.25 Any degree of sleepiness which interfered with daily activities was regarded as significant for the purpose of our study. The patients were recruited from the Respiratory Clinic, Prince of Wales Hospital, Hong Kong.
The study was approved by the ethics committee of the Chinese University of Hong Kong and appropriate informed written consent was obtained from the subjects.
Sleep assessment
Overnight diagnostic PSG (Healthdyne Alice 4, USA) was performed in all subjects; electroencephalography (EEG), electro‐oculography, submental electromyography (EMG), bilateral anterior tibial EMG, electrocardiography, chest and abdominal wall movement were recorded by inductance plethysmography and airflow was measured by a nasal pressure transducer (PTAF2, Pro‐Tech, Woodinville, WA, USA) supplemented by an oral thermistor and finger pulse oximetry, as described in our previous studies.26,27,28 Sleep stages were scored according to the standard criteria of Rechtshaffen and Kales.29 Apnoea was defined as cessation of airflow for >10 seconds and hypopnoea as a reduction in airflow of ⩾50% for >10 seconds plus an oxygen desaturation of >3% or an arousal. An arousal was scored if there was a 3 second or longer abrupt shift in EEG frequency to alpha or theta or >16 Hz following at least 10 seconds of sleep, and if arising in REM there must be a rise in EMG tone.30
Following confirmation of OSA,25 each patient was interviewed by the physician on duty and invited to participate in the study. The patients who consented were randomised into two groups to receive nasal therapeutic or subtherapeutic CPAP in a balanced block design after completion of recording baseline 24 hour ambulatory BP.
Ambulatory blood pressure monitoring (ABPM)
All patients with OSA were fitted with the Ultralite ABPM device (Spacelabs Medical, Redmond, WA, USA) via an arm cuff as outpatients during normal activities and monitored for 48 hours before starting treatment with therapeutic or subtherapeutic CPAP.18 The arm circumference of the patients was measured at the biceps level and an appropriate sized arm cuff was applied. The machine was programmed for cuff inflation measurement every 30 minutes for 48 hours, and it could only be removed for bathing. Patients were asked to abstain from caffeine containing products during this time but to continue their normal daily activities. They were instructed to record the time they retired to bed and the subsequent time of waking in order to identify the sleep period. Data gathered before 18.00 hours on the second evening were discarded to allow for acclimatisation, and the analysis was performed with the second 24 hours of data.18 Data were manually checked for artifacts by an investigator who was blinded to the treatment status of the patient. Forty eight hours before the end of the therapeutic or subtherapeutic CPAP at 3 months, the patients were fitted with the ABPM machine and the same procedure repeated.
Therapeutic v subtherapeutic CPAP treatment
Patients in the therapeutic or subtherapeutic CPAP treatment arm were given a basic CPAP education programme by a respiratory nurse supplemented by an education brochure and a video.28 A short trial of CPAP therapy with the Autoset (Resmed, Sydney, Australia) CPAP device was given for approximately 30 minutes for acclimatisation in the afternoon. Attended overnight CPAP titration was performed with the Autoset autotitrating device for those randomised to the therapeutic CPAP arm. The CPAP level for each patient in the therapeutic CPAP arm was set at the minimum pressure needed to abolish snoring, obstructive respiratory events, and airflow limitation for 95% of the night as determined by the overnight CPAP titration study.26,27,28
Patients randomised to the subtherapeutic CPAP arm after PSG had the CPAP units set at a low pressure (4 cm H2O) for home use.22 Patients in the therapeutic and subtherapeutic CPAP arms were prescribed CPAP devices with time counters recording machine run time.
All patients were followed up at the respiratory clinic at 1 month and 3 months for any problem related to use of CPAP, and CPAP usage was measured from the time counter at the end of the study. Although the two different treatment arms were explained in the patient information, the CPAP naïve patients were not aware of whether they received therapeutic or subtherapeutic CPAP during the study period. The investigator responsible for randomisation of patients to the different treatment arms did not participate in outcome assessments which were conducted by a different team of investigators who were not aware of the randomisation status of the patients. On completion of the study (at 3 months), all patients in the subtherapeutic CPAP arm were readmitted overnight for attended AutoSet CPAP titration to establish their long term therapeutic pressure.
Outcome assessment
The primary outcome of interest was the change in mean 24 hour arterial BP (1/3 systolic plus 2/3 diastolic) at 3 months. The secondary outcome of interest included changes in systolic and diastolic BP, changes in mean BP awake and asleep, and whether any change in BP was related to the baseline hypertensive status and CPAP compliance over 3 months.
Before starting nasal therapeutic or subtherapeutic CPAP, all patients had assessment of subjective sleepiness with the Epworth Sleepiness Scale (ESS).31 The ESS is a questionnaire specific to symptoms of daytime sleepiness and patients are asked to score the likelihood of falling asleep in eight different situations with different levels of stimulation, adding up to a total score of 0–24.31 All patients had to complete the ESS at baseline and 3 months after treatment.
The inclusion criteria for the study were age 20–80 years and AHI ⩾5/hour on PSG with symptoms of OSA as described previously.25 Patients with hypertension were eligible to enter and continue the study as long as there was no change in antihypertensive medications during the study period. Hypertension was defined as a previously documented BP of >140/90 mm Hg on at least two occasions or on antihypertensive medications. Patients with problems staying awake during driving, professional drivers, shift workers, and those with recent myocardial infarction, unstable angina, or underlying malignancy were excluded from the study.
Sample size calculation
The sample size was estimated by the Power Analysis and Sample Size for Windows software (PASS2000, NCSS, Kaysville, UH, USA). Based on data by Becker et al,22 group sample sizes of 42 and 42 achieved 90% power to detect a difference of −8.6 mm Hg between the null hypothesis that both groups had a change in mean BP of 0.6 mm Hg and the alternative hypothesis that the mean of the treatment group was 9.2 mm Hg with estimated group standard deviations of 12.0 mm Hg and with a significance level of 0.05 using a two‐sided two‐sample t test. Assuming a dropout rate of 20%, a total sample size of 50 patients for each group would be adequate.
Data analysis
The data are presented as mean (SE) unless otherwise stated. For comparison between controls and CPAP treatment group at each time point, an unpaired t test was used for normally distributed variables and a Mann‐Whitney test for non‐normally distributed variables. To compare the measurements before and after subtherapeutic or therapeutic CPAP treatment, a paired t test was used for normally distributed variables and Wilcoxon's signed rank test for non‐normally distributed variables. Data analysis was performed with a commercially available statistical analysis software package (SPSS 11.5 for Windows, SPSS Inc, Chicago, IL, USA).
Results
Study profile
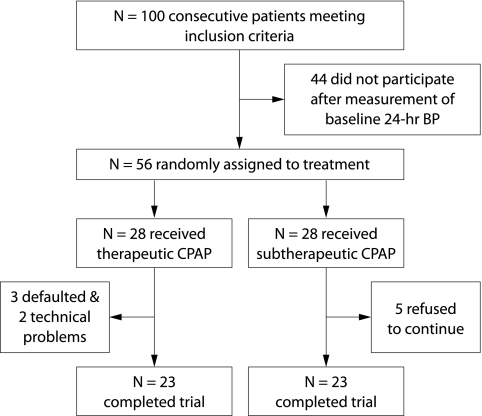
The study profile is described in fig 1. There were no significant differences in the baseline demographics, OSA severity, and BP parameters between patients who agreed (n = 56) and other eligible patients who refused to take part in the study (n = 44, table 1).
Figure 1 Study profile. Of 100 consecutive patients eligible for the study, 44 did not enter the randomisation process because of refusal to participate (n = 31), being on shift work (n = 7), or being professional drivers (n = 6). 28 patients were randomised into each treatment arm. Five patients in the subtherapeutic arm refused to continue treatment at the 1 month follow up as they found the treatment uncomfortable. Technical failure of ambulatory BP recording was noted in two patients in the active CPAP arm and another three defaulted their appointments for ambulatory BP measurement.
Table 1 Comparison of baseline parameters between patients who were randomised and those who refused to participate in the study.
| Randomised (n = 56) | Refused (n = 44) | p value | |
|---|---|---|---|
| M/F | 43/13 | 35/9 | 0.811† |
| Age (years) | 50.8 (1.7) | 48.8 (1.4) | 0.281 |
| Neck circumference (cm) | 38.5 (0.4) | 39.2 (0.8) | 0.454 |
| Waist circumference (cm) | 94.1 (1.4) | 96.1 (2.4) | 0.447 |
| BMI (kg/m2) | 27.2 (0.5) | 28.1 (0.7) | 0.239 |
| ESS score | 11.1 (0.7) | 9.8 (0.8) | 0.230 |
| Smoking status | 0.129* | ||
| Never | 43 | 20 | |
| Quit | 8 | 8 | |
| Current | 5 | 7 | |
| Alcohol consumption | 0.101* | ||
| Never | 34 | 15 | |
| Quit | 4 | 1 | |
| Current | 18 | 19 | |
| Hypertension | 0.581† | ||
| Yes | 28 | 21 | |
| No | 28 | 21 | |
| AHI (events/hour) | 31.2 (2.2) | 38.4 (4.0) | 0.093 |
| Minimum Sao2 (%) | 74.6 (1.9) | 72.6 (3.1) | 0.556 |
| Mean Sao2 (%) | 93.7 (0.4) | 92.9 (0.9) | 0.363 |
| Arousal index | 31.8 (2.4) | 27.7 (3.2) | 0.297 |
| Sao2 <90 (TST %) | 8.4 (2.4) | 2.9 (1.9) | 0.093 |
| TST (min) | 387.6 (16.1) | 385.3 (27.0) | 0.940 |
| Mean 24 hour BP (mm Hg) | |||
| Systolic | 123.7 (1.8) | 129.3 (2.3) | 0.054 |
| Diastolic | 80.9 (1.2) | 74.1 (1.4) | 0.091 |
| MAP | 95.2 (1.3) | 98.7 (1.5) | 0.087 |
| Wake time BP | |||
| Systolic | 127.8 (1.8) | 132.0 (2.3) | 0.150 |
| Diastolic | 83.6 (1.2) | 85.6 (1.4) | 0.306 |
| MAP | 98.1 (1.5) | 100.2 (1.6) | 0.301 |
| Sleep time BP | |||
| Systolic | 115.7 (2.0) | 119.1 (2.6) | 0.291 |
| Diastolic | 74.8 (1.4) | 76.7 (1.5) | 0.364 |
| MAP | 89.1 (1.5) | 90.7 (1.7) | 0.475 |
AHI, apnoea‐hypopnoea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; Sao2, oxygen saturation; TST, total sleep time; Sao2 <90 (TST %) = % of total sleep time with oxygen saturation <90%; MAP, mean arterial blood pressure.
Values are expressed as mean (SE).
*χ2 test.
†Fisher's exact test.
There were no significant differences in the baseline characteristics between the therapeutic and subtherapeutic CPAP groups (n = 28 in each group, table 2). In addition, there were no significant differences in the baseline demographics, OSA severity, and BP parameters between patients who completed the study (n = 46) and those who did not (n = 10, data shown in table S1 available online only at http://www.thoraxjnl.com.supplemental).
Table 2 Comparisons of baseline parameters between therapeutic and subtherapeutic CPAP groups.
| Therapeutic CPAP (n = 28) | Subtherapeutic CPAP (n = 28) | p value | |
|---|---|---|---|
| M/F | 22/6 | 21/7 | 1.000† |
| Age (years) | 50.3 (1.6) | 51.2 (1.8) | 0.718 |
| Neck circumference (cm) | 39.1 (0.6) | 37.9 (0.6) | 0.163 |
| Waist circumference (cm) | 95.2 (1.8) | 92.9 (2.1) | 0.406 |
| Hip circumference (cm) | 100.7 (2.0) | 102.0 (1.7) | 0.623 |
| BMI (kg/m2) | 27.5 (0.6) | 26.9 (0.7) | 0.546 |
| ESS score | 10.7 (1.0) | 11.6 (1.0) | 0.543 |
| Smoking status | 0.894* | ||
| Never | 21 | 22 | |
| Quit | 4 | 4 | |
| Current | 3 | 2 | |
| Alcohol consumption | 0.543* | ||
| Never | 17 | 17 | |
| Quit | 3 | 1 | |
| Current | 8 | 10 | |
| Hypertension | 0.181† | ||
| Yes | 17 | 11 | |
| No | 11 | 17 | |
| AHI (events/hour) | 32.9 (3.2) | 29.5 (3.1) | 0.455 |
| Minimum Sao2 (%) | 73.4 (2.5) | 75.8 (2.8) | 0.531 |
| Mean Sao2 (%) | 93.0 (0.6) | 94.4 (0.4) | 0.057 |
| Arousal index | 33.2 (3.6) | 30.3 (3.1) | 0.550 |
| Sao2 <90 (TST %) | 5.6 (2.8) | 11.3 (3.8) | 0.231 |
| Mean 24 hour BP (mm Hg) | |||
| Systolic | 125.4 (2.6) | 122.0 (2.7) | 0.359 |
| Diastolic | 81.8 (1.9) | 80.0 (1.7) | 0.483 |
| MAP | 96.2 (1.8) | 94.1 (2.0) | 0.417 |
| Wake time BP | |||
| Systolic | 128.6 (2.6) | 127.2 (2.7) | 0.689 |
| Diastolic | 83.7 (1.9) | 83.7 (1.7) | 0.966 |
| MAP | 98.3 (1.8) | 97.9 (1.9) | 0.857 |
| Sleep time BP | |||
| Systolic | 117.7 (2.9) | 113.9 (3.0) | 0.314 |
| Diastolic | 75.8 (2.1) | 74.1 (2.0) | 0.478 |
| MAP | 90.6 (2.1) | 87.8 (2.3) | 0.317 |
For definition of abbreviations, see footnote to table 1.
Values are expressed as mean (SE).
*χ2 test.
†Fisher's exact test.
Demographic data and ESS scores
Only 23 patients in each arm completed the treatment protocol and outcome measurements at the end of the 3 month study. There were no significant differences in the baseline demographics, OSA severity, and BP parameters between the two treatment groups (table 3). In the therapeutic CPAP group the ESS ranged from 3 to 18 with 10 subjects (43.5%) having an ESS >10. In the subtherapeutic CPAP group the ESS ranged from 2 to 20 with 14 (60.9%) having an ESS >10. After 3 months of treatment there was no significant change in patient demographics in each treatment arm (table 4). However, there were significant improvements in ESS scores in both groups with no significant difference between them (mean (SE) change in ESS −3.2 (0.9) v −3.1 (1.1) in the therapeutic and subtherapeutic CPAP groups, respectively), with a mean difference between changes in ESS of −0.04 (95% CI −2.94 to 2.86, p = 0.979).
Table 3 Comparisons of baseline characteristics and CPAP usage between therapeutic and subtherapeutic CPAP groups who completed the treatment protocol.
| Therapeutic CPAP (n = 23) | Subtherapeutic CPAP (n = 23) | p value | |
|---|---|---|---|
| M/F | 19/4 | 18/5 | 1.000† |
| Age (years) | 50.2 (1.7) | 51.4 (2.1) | 0.666 |
| Neck circumference (cm) | 39.2 (0.5) | 38.2 (0.7) | 0.266 |
| Waist circumference (cm) | 95.2 (2.1) | 93.8 (2.2) | 0.646 |
| BMI (kg/m2) | 27.4 (0.7) | 26.9 (0.8) | 0.670 |
| ESS (0–24) | 10.2 (1.0) | 11.2 (1.1) | 0.522 |
| Smoking status | 0.918* | ||
| Never | 18 | 17 | |
| Quit | 3 | 4 | |
| Current | 2 | 2 | |
| Alcohol consumption | 0.193* | ||
| Never | 13 | 14 | |
| Quit | 3 | 0 | |
| Current | 7 | 9 | |
| Hypertension | 0.139† | ||
| Yes | 15 | 9 | |
| No | 8 | 14 | |
| AHI | 31.3 (3.3) | 29.1 (3.6) | 0.652 |
| Minimum Sao2 (%) | 75.6 (2.8) | 75.1 (3.2) | 0.910 |
| Mean Sao2 (%) | 93.2 (0.8) | 94.3 (0.4) | 0.193 |
| Arousal index | 34.1 (3.8) | 32.0 (3.2) | 0.671 |
| Sao2 <90 (TST %) | 7.1 (3.5) | 12.1 (4.5) | 0.383 |
| Mean 24 hour BP | |||
| Systolic | 125.9 (3.0) | 122.0 (3.0) | 0.870 |
| Diastolic | 82.5 (2.2) | 79.6 (2.2) | 0.377 |
| MAP | 96.8 (2.1) | 93.9 (2.1) | 0.347 |
| Wake time BP | |||
| Systolic | 129.1 (3.0) | 127.7 (3.0) | 0.752 |
| Diastolic | 84.3 (2.2) | 83.6 (2.1) | 0.817 |
| MAP | 98.9 (2.1) | 98.1 (2.1) | 0.870 |
| Sleep time BP | |||
| Systolic | 118.3 (3.3) | 113.7 (3.3) | 0.337 |
| Diastolic | 76.9 (2.5) | 73.6 (2.2) | 0.336 |
| MAP | 91.3 (2.5) | 87.5 (2.5) | 0.682 |
| CPAP usage (hours/night) over 3 months | 5.1 (0.4) | 2.6 (0.4) | 0.000 |
| AutoSet CPAP titration pressure (cm H2O) | 10.7 (0.4) | 9.6 (0.5) | 0.099 |
For definition of abbreviations, see footnote to table 1.
Values are expressed as mean (SE).
*χ2 test.
†Fisher's exact test.
Patients in the subtherapeutic CPAP group had AutoSet CPAP titration study performed after completion of the 3 month study.
Table 4 Comparison of demographic data and ESS before and after 3 months of treatment.
| Therapeutic CPAP (n = 23) | Subtherapeutic CPAP (n = 23) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | p value* | Baseline | 3 months | p value* | |
| Body weight (kg) | 74.0 (2.5) | 74.9 (2.2) | 0.235 | 71.6 (2.3) | 72.7 (2.1) | 0.391 |
| Neck circumference (cm) | 39.2 (0.5) | 39.3 (0.6) | 0.710 | 38.2 (0.7) | 38.1 (0.6) | 0.861 |
| Waist circumference (cm) | 95.2 (2.1) | 94.6 (1.6) | 0.745 | 93.8 (2.2) | 94.8 (2.4) | 0.513 |
| BMI (kg/m2) | 27.4 (0.7) | 27.7 (0.7) | 0.184 | 26.9 (0.8) | 27.4 (0.8) | 0.309 |
| ESS | 10.2 (1.0) | 7.0 (1.0) | 0.002 | 11.2 (1.1) | 8.1 (1.0) | 0.011 |
For definition of abbreviations, see footnote to table 1.
Values are expressed as mean (SE).
*Paired t test.
Antihypertensive medications
There were no changes in antihypertensive medications or dosages during the study period. In the therapeutic CPAP group, six patients were on one medication (β‐blocker, n = 2; calcium channel blocker, n = 4), two were on two medications (β‐blocker + calcium channel blocker), three were on three medications (α‐blocker + β‐blocker + calcium channel blocker, n = 1; β‐blocker + calcium channel blocker + diuretic, n = 1; α‐blocker + β‐blocker + diuretic, n = 1), and one patient was on four medications (angiotensin converting enzyme inhibitor + α‐blocker + β‐blocker + methyldopa). In the subtherapeutic CPAP group, one patient was on one medication (β‐blocker), three were on two medications (β‐blocker + diuretic, n = 1; calcium channel blocker + diuretic, n = 1; β‐blocker + calcium channel blocker, n = 1), and one patient was on three drugs (angiotensin converting enzyme inhibitor + β‐blocker + diuretic).
CPAP usage and levels
Patients receiving therapeutic CPAP were more compliant than those receiving subtherapeutic CPAP. The latter had AutoSet CPAP titration performed after completion of outcome assessments at 3 months to determine the CPAP level for long term use. No significant difference in CPAP levels required to abolish snoring, obstructive events, and airflow limitation was found between the two groups (table 3).
Changes in 24 hour BP between therapeutic and subtherapeutic CPAP groups
At baseline there were no significant differences between the two groups in mean 24 hour BP, wake time BP, and sleep time BP (table 3).Three months after therapeutic CPAP treatment there were significant reductions in 24 hour diastolic BP and sleep time mean BP, with trends for significant reductions in sleep time systolic BP, sleep time diastolic BP, and 24 hour mean BP. In contrast, there were no significant changes in any of the BP parameters in the subtherapeutic CPAP group.
When changes in the BP parameters following 3 months of treatment in the two groups were compared, there were significant reductions in 24 hour mean BP (−3.8 mm Hg), 24 hour diastolic BP (−3.5 mm Hg), sleep time systolic BP (−6.3 mm Hg), and sleep time mean BP (−4.9 mm Hg) in the therapeutic CPAP group compared with the subtherapeutic group (table 5).
Table 5 Changes in blood pressure parameters in therapeutic and subtherapeutic CPAP groups.
| Therapeutic CPAP (n = 23) | Therapeutic CPAP (n = 23) | Subtherapeutic CPAP (n = 23) | Subtherapeutic CPAP (n = 23) | Difference (95% CI) in BP changes (mm Hg) | p value ANOVA | |
|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |||
| Sleep time BP | ||||||
| Systolic | 118.3 (3.3) | 114.2 (2.8) | 113.7 (3.3) | 115.9 (2.9) | −6.3 (−11.8 to −0.7) | 0.028* |
| Diastolic | 76.9 (2.5) | 73.9 (2.1) | 73.6 (2.2) | 74.5 (1.8) | −3.8 (−8.0 to 0.4) | 0.078 |
| MAP | 91.3 (2.5) | 87.8 (2.1) | 87.5 (2.5) | 88.7 (2.0) | −4.9 (−9.2 to −0.4) | 0.033* |
| Wake time BP | ||||||
| Systolic | 129.1 (3.0) | 126.8 (3.0) | 127.7 (3.0) | 128.0 (3.4) | −2.5 (−8.2 to 3.2) | 0.376 |
| Diastolic | 84.3 (2.1) | 82.3 (2.0) | 83.6 (2.1) | 83.4 (1.9) | −1.8 (−5.3 to 1.8) | 0.317 |
| MAP | 98.9 (2.1) | 96.9 (2.1) | 98.1 (2.1) | 98.3 (2.4) | −2.2 (−6.2 to 1.9) | 0.293 |
| Mean 24 hour BP | ||||||
| Systolic | 125.9 (3.0) | 123.5 (2.8) | 122.0 (3.0) | 120.2 (5.6) | −0.4 (−10.7 to 9.8) | 0.928 |
| Diastolic | 82.5 (2.2) | 80.1 (2.0) | 79.6 (2.2) | 80.7 (1.8) | −3.5 (−6.6 to −0.5) | 0.025* |
| MAP | 96.8 (2.1) | 94.4 (2.0) | 93.9 (2.1) | 95.2 (2.1) | −3.8 (−7.2 to −0.2) | 0.037* |
Values are expressed as mean (SE).
MAP, mean arterial blood pressure.
* Statistically significant.
Subgroup analysis was performed to compare changes in BP parameters between hypertensive patients in the therapeutic CPAP group (n = 15) and hypertensive patients in the subtherapeutic CPAP group (n = 9). While no significant differences in BP changes were noted for sleep time and wake time BP parameters, there were trends for significant differences in changes in mean (SE) 24 hour BP parameters between the two groups (data shown in table S2 available online only at http://www.thoraxjnl.com/supplemental):
24 hour mean BP: −3.5 (1.8) v 2.2 (2.2) (95% CI −11.7 to 0.5 mm Hg, p = 0.068);
24 hour systolic BP: −3.8 (2.7) v 4.8 (3.2) (95% CI −17.5 to 0.2 mm Hg, p = 0.056);
24 hour diastolic BP: −3.2 (1.6) v 1.5 (1.9) (95% CI −10.0 to 0.6 mmHg, p = 0.077).
Further subgroup analysis was performed to compare changes in BP parameters between hypertensive (n = 15) and normotensive patients (n = 8) in the therapeutic CPAP group and between hypertensive (n = 9) and normotensive patients (n = 14) in the subtherapeutic CPAP group. No significant differences were found in changes in BP parameters between the two groups (data shown in tables S3 and S4 available online only at http://www.thoraxjnl.com.supplemental).
Subgroup analysis was performed to compare changes in BP parameters in patients with good CPAP compliance (n = 13 with CPAP usage ⩾5 hour/night) against those with CPAP usage <5 hours/night (n = 10) in the therapeutic CPAP group. There were no significant differences in changes in BP parameters between the two groups.
The changes in BP parameters in patients with higher AHI defined as AHI ⩾ median AHI for the group, that is ⩾27.4/hour (n = 12) were compared against those with AHI <27.4/hour (n = 11) in the therapeutic CPAP group. No significant differences in changes in BP parameters were seen between the two groups.
Discussion
This study has shown that 12 weeks of nasal therapeutic CPAP treatment led to significant though modest decreases in 24 hour mean BP and 24 hour diastolic BP of 3.8 and 3.5 mm Hg, respectively, in mildly sleepy patients with OSA relative to the controls. Not surprisingly, there were also reductions in sleep time systolic BP and sleep time mean BP of 6.3 and 4.9 mm Hg, respectively, in those receiving therapeutic CPAP compared with the controls. Our definition of OSA syndrome for this study was based on that of the American Academy of Sleep Medicine, which took into consideration other symptoms apart from daytime sleepiness.25 Our patients had a relatively mild degree of subjective sleepiness with a mean ESS score of 10–11, although they had moderately severe OSA with a median AHI of 27.9/hour and a mean trough Sao2 of 75% during sleep. The mean (SD) ESS scores in normal subjects compared with patients with OSA in a study in Hong Kong were 7.5 (3.0) and 13.2 (4.7), respectively.32
There are conflicting data as to whether nasal CPAP can reduce systemic BP in patients with OSA.17,18,19,20,21,22,23,24 Randomised placebo controlled trials of the effect of CPAP over 4 weeks on 24 hour BP have generally shown modest though significant decreases in BP in sleepy patients with OSA.18,21 Faccenda et al18 reported a reduction in 24 hour diastolic BP of 1.5 mm Hg across all sleepy patients (n = 68, mean AHI 35/hour and median ESS 15) with the greatest falls between 02.00 and 10.00 hours, but no significant change in systolic BP after 4 weeks of nasal CPAP treatment compared with oral placebo. Those using CPAP for >3.5 hours/night had a decrease in 24 hour diastolic BP of 1.9 mm Hg, whereas those with >20 episodes of 4% desaturations/hour had reductions in 24 hour diastolic and 24 hour systolic BP of 5 mm Hg and 4 mm Hg, respectively.18 Pepperell et al21 reported a 3.3 mm Hg reduction in 24 hour mean BP and 24 hour diastolic BP among sleepy patients (mean ESS 16) with OSA in the therapeutic (n = 59) v the sub‐therapeutic CPAP arm (n = 59) after 4 weeks, and more beneficial effect of CPAP on BP was seen in those with an oxygen desaturation index (ODI) of >33 events/hour.
In contrast, Barnes et al20,23 were unable to show any significant change in 24 hour BP after 8–12 weeks of CPAP treatment in mildly sleepy patients (mean ESS 11.2 and 10.7, respectively) whose AHI was 5–30/hour, whereas Barbe et al19 reported no effect on BP in non‐sleepy patients (mean ESS 7) with AHI ⩾30/hour randomised to receive either CPAP (n = 29) or sham CPAP (n = 25) over 6 weeks. Recently, Robinson et al24 reported no significant change in BP in non‐sleepy OSA patients (n = 35, median ESS 5.3 and ODI 28/hour) randomised to therapeutic or subtherapeutic CPAP treatment for 4 weeks. Thus, it seems that nasal CPAP has no favourable effect on systemic BP in short to medium term studies of patients who are either not sleepy or mildly sleepy.
On the more severe end of the spectrum, Becker et al22 have shown that sleepy patients with severe OSA (mean ESS 14 and AHI 63/hour) in the active CPAP treatment arm (n = 16) achieved a reduction in 24 hour mean BP of 9.9 mm Hg over a period of 9 weeks compared with controls (n = 16) on subtherapeutic CPAP of 3 or 4 cm H2O. It is of interest to note that Becker et al22 used a continuous non‐invasive Portapres (photoplethysmograph) technique to measure BP which might not cause arousal from sleep, whereas others17,18,19,20,21,23,24 (including our study) used cuff inflation techniques which might induce frequent arousals and hence elevation of BP.33,34 Despite this technical limitation, we have shown that nasal CPAP led to reductions in 24 hour diastolic BP and 24 hour mean arterial BP of 3.5 and 3.8 mm Hg, respectively, in mildly sleepy patients with OSA relative to the controls. There were trends for similar differences in changes in 24 hour BP parameters when the hypertensive patients in the two treatments groups were compared. From large prospective studies using antihypertensive medications in non‐OSA populations, a fall in the BP of such magnitude would be expected to be associated with a reduction in the risk of stroke of about 20% and a reduction in the risk of coronary artery disease of about 15%.35
It is interesting to note that the baseline mean ESS score of our OSA patients was similar to those in the two negative crossover studies by Barnes et al20,23 over 8 and 12 weeks, respectively, but our patients had higher mean CPAP usage (5.1 v 3.5–3.6 hours/day). More recently, Norman et al36 have shown that 2 weeks of nasal CPAP reduced daytime mean and diastolic BP by 3 mm Hg, with similar magnitudes of decline in night time systolic, mean and diastolic BP as in our study, in mildly sleepy OSA patients (n = 18, mean AHI 66/hour and ESS 12) with good CPAP compliance (mean use 6.7 hours/night). In their sham CPAP group receiving 0.5 cm H2O on end expiration there was a 3 mm Hg rise in night time systolic BP whereas no significant BP changes were observed in other controls given supplemental oxygen alone.36
The comparable improvement in ESS scores in both treatment groups in this study is intriguing, given the relatively low CPAP usage in the subtherapeutic CPAP group. The improvement in ESS could be due to partial treatment effect, as Becker et al22 have shown that subtherapeutic CPAP at 3–4 cm H2O could reduce AHI by half and improve sleep architecture and oxygenation although BP did not decrease. In addition, the lack of significant change in body weight and BMI in the subtherapeutic CPAP group in our study over 3 months suggests that improvement in subjective sleepiness might be due to improvement in sleep hygiene and possibly a placebo effect. An oral placebo could reduce the mean ESS score by 2.5,18 whereas subtherapeutic CPAP reduced ESS by 2–5.2 from baseline values after treatment for 4–9 weeks.16,22 It remains unclear what role the symptoms of OSA play in the responsiveness of BP to CPAP treatment.
While the main indication of CPAP treatment in OSA is to relieve disabling symptoms, especially daytime sleepiness,1,37,38 even a modest reduction in systemic BP with nasal CPAP should be regarded as a bonus benefit, especially with the growing evidence of cardiovascular consequences related to OSA.5,6,7,8,9,10,11,12,13,39 In patients who are not able to tolerate nasal CPAP, a dental appliance in the form of a mandibular advancement device can improve symptoms40 and reduce 24 hour diastolic BP by 1.8 mm Hg after 4 weeks of treatment.41
This study has several limitations. Firstly, only 46 out of 56 patients completed the treatment protocol and data analyses on changes in BP parameters were performed for those who had completed the study, rather than on an intention to treat basis. This might introduce potential selection bias although there were no significant differences in baseline demographic data, ESS, OSA severity, and BP parameters between those who completed the study and those who did not. Secondly, the study was underpowered and the small sample size made it difficult to perform meaningful subgroup analysis of the effects of AHI, hypertension, and CPAP usage on 24 hour BP. We were unable to recruit sufficient subjects because many of the patients with OSA that we had screened during the study period were working actively and could not afford extra time off work to undergo serial 24 hour BP monitoring and other outcome measures assessment. Apart from subjective sleepiness, we did not perform objective assessment of sleepiness due to limited resources. Lastly, as experienced by other investigators,16,24 CPAP usage in the subtherapeutic CPAP arm was lower than in the therapeutic arm so a full placebo benefit might not be obtained at the end of the study limbs when the key measurements were made. This is a practical and probably inevitable problem in the design of any longer term study of the efficacy of CPAP, and was the reason why other studies have used an oral placebo tablet instead.18,20,23,42
In summary, this study has shown that 12 weeks of nasal therapeutic CPAP treatment led to modest reductions in 24 hour mean BP and 24 hour diastolic BP of 3.8 and 3.5 mm Hg, respectively, in mildly sleepy patients with moderately severe OSA relative to the controls. Further studies with larger sample sizes are needed to compare the BP lowering effects of CPAP over a longer period in hypertensive and normotensive patients with OSA.
Further details are given in tables S1–S4 available online at http://www.thoraxjnl.com.supplemental.
Supplementary Material
Acknowledgements
The authors thank all the nurses at the Division of Respiratory Medicine for their technical support for this study.
Abbreviations
ABPM - ambulatory blood pressure monitoring
AHI - apnoea‐hypopnoea index
CPAP - continuous positive airway pressure
ESS - Epworth Sleepiness Scale
ODI - oxygen desaturation index
OSA - obstructive sleep apnoea
PSG - polysomnography
Footnotes
Funded by the Chinese University of Hong Kong Direct Grants #2040981 and #2041204.
Competing interests: none declared.
Further details are given in tables S1–S4 available online at http://www.thoraxjnl.com.supplemental.
References
- 1.Engleman H M, Douglas N J. Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax 200459618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J.et al The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med 19933281230–1235. [DOI] [PubMed] [Google Scholar]
- 3.Ip M S, Lam B, Lauder I J.et al A community study of sleep‐disordered breathing in middle‐aged Chinese men in Hong Kong. Chest 200111962–69. [DOI] [PubMed] [Google Scholar]
- 4.Hui D S, Chan J K, Ko F W.et al Prevalence of snoring and sleep‐disordered breathing in a group of commercial bus drivers in Hong Kong. Intern Med J 200232149–157. [DOI] [PubMed] [Google Scholar]
- 5.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea as a risk factor for hypertension: population study. BMJ 2000320479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieto F J, Young T B, Lind B K.et al Association of sleep‐disordered breathing, sleep apnea and hypertension in a large community‐based study. JAMA 20002831829–1836. [DOI] [PubMed] [Google Scholar]
- 7.Peppard P E, Young T, Palta M.et al Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med 20003421378–1384. [DOI] [PubMed] [Google Scholar]
- 8.Davies C W, Crosby J H, Mullins R L.et al Case‐control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax 200055736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahar E, Whitney C W, Redline S.et al Sleep‐disordered breathing and cardiovascular disease. Cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 200116319–25. [DOI] [PubMed] [Google Scholar]
- 10.Peker Y, Hedner J, Norum J.et al Increased incidence of cardiovascular disease in middle‐aged men with obstructive sleep apnea. Am J Respir Crit Care Med 2002166159–165. [DOI] [PubMed] [Google Scholar]
- 11.Marin J M, Carrizo S J, Vicente E.et al Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 20053651046–1053. [DOI] [PubMed] [Google Scholar]
- 12.Gami A S, Howard D E, Olson E J.et al Day‐night pattern of sudden death in obstructive sleep apnea. N Engl J Med 20053521206–1214. [DOI] [PubMed] [Google Scholar]
- 13.Yaggi H K, Concato J, Kernan W N.et al Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 20053532034–2041. [DOI] [PubMed] [Google Scholar]
- 14.Doherty L S, Kiely J L, Swan V.et al Long‐term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 20051272076–2084. [DOI] [PubMed] [Google Scholar]
- 15.Engleman H, Martin S, Deary I.et al Effect of continuous positive airway pressure treatment on daytime function in sleep apnea/hypopnea syndrome. Lancet 1994343572–575. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson C, Davies R, Mullins R.et al Comparison of therapeutic and sub‐therapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomized prospective parallel trial. Lancet 19993532100–2105. [DOI] [PubMed] [Google Scholar]
- 17.Dimsdale J E, Loredo J S, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension 200035144–147. [DOI] [PubMed] [Google Scholar]
- 18.Faccenda J F, Mackay T W, Boon N A.et al Randomized placebo‐controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea‐hypopnea syndrome. Am J Respir Crit Care Med 2001163344–348. [DOI] [PubMed] [Google Scholar]
- 19.Barbe F, Mayoralas L R, Duran J.et al Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomised, controlled trial. Ann Intern Med 20011341015–1023. [DOI] [PubMed] [Google Scholar]
- 20.Barnes M, Houston D, Worsnop C J.et al A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med 2002165773–780. [DOI] [PubMed] [Google Scholar]
- 21.Pepperell J C, Ramdassingh‐Dow S, Crosthwaite N.et al Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomized parallel trial. Lancet 2002359204–210. [DOI] [PubMed] [Google Scholar]
- 22.Becker H F, Jerrentrup A, Ploch T.et al Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 200310768–73. [DOI] [PubMed] [Google Scholar]
- 23.Barnes M, McEvoy R D, Banks S.et al Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 2004170656–664. [DOI] [PubMed] [Google Scholar]
- 24.Robinson G V, Smith D M, Langford B A.et al CPAP does not reduce blood pressure in non‐sleepy hypertensive OSA patients. Eur Respir J 2006271229–1235. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Sleep Medicine Task Force Sleep‐related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 199922667–690. [PubMed] [Google Scholar]
- 26.Fung J W, Li T S, Choy D K.et al Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest 2002121422–429. [DOI] [PubMed] [Google Scholar]
- 27.Hui D S, Choy D K, Wong L K.et al Prevalence of sleep‐disordered breathing and CPAP compliance. Results in a group of Chinese patients post first‐ever ischemic stroke. Chest 2002122852–860. [DOI] [PubMed] [Google Scholar]
- 28.Hui D S, Chan J K, Choy D K.et al Effects of augmented CPAP education and support on compliance and outcome in a Chinese population. Chest 20001171410–1416. [DOI] [PubMed] [Google Scholar]
- 29.Rechtschaffen A, Kales A.A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service, Brain Information Institute, University of California, 1968
- 30.American Sleep Disorders Association ( A S D A ) EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 199215173–184. [PubMed] [Google Scholar]
- 31.Johns M W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 199114540–545. [DOI] [PubMed] [Google Scholar]
- 32.Chung K F. Use of the Epworth Sleepiness Scale in Chinese patients with obstructive sleep apnea and normal hospital employees. J Psychosom Res 200049367–372. [DOI] [PubMed] [Google Scholar]
- 33.Heude E, Bourgin P, Feigel P.et al Ambulatory monitoring of blood pressure disturbs sleep and raises systolic pressure at night in patients suspected of suffering from sleep‐disordered breathing. Clin Sci (Lond) 19969145–50. [DOI] [PubMed] [Google Scholar]
- 34.Davies R J, Jenkins N E, Stradling J R. Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ 1994308820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacMahon S, Peto R, Cutler J.et al Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990335765–774. [DOI] [PubMed] [Google Scholar]
- 36.Norman D, Loredo J S, Nelesen R A.et al Effects of continuous positive airway pressure versus supplemental oxygen on 24‐hr ambulatory blood pressure. Hypertension 2006471–6. [DOI] [PubMed] [Google Scholar]
- 37.Engleman H M, Martin S E, Deary I J.et al The effect of continuous positive airway pressure therapy on daytime function in the sleep apnoea/hypopnoea syndrome. Lancet 1994343572–575. [DOI] [PubMed] [Google Scholar]
- 38.George C F. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 200156508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shamsuzzaman A S, Gersh B J, Somers V K. Obstructive sleep apnea‐ Implications for cardiac and vascular disease. JAMA 20032901906–1914. [DOI] [PubMed] [Google Scholar]
- 40.Gotsopoulos H, Chen C, Qian J.et al Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med 2002166743–748. [DOI] [PubMed] [Google Scholar]
- 41.Gotsopoulos H, Kelly J J, Cistulli P A. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep 200427934–941. [DOI] [PubMed] [Google Scholar]
- 42.Douglas N J, Engleman H M, Faccenda J F.et al The science of designing ethical CPAP trials. Am J Respir Crit Care Med 2002165132–134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.