Abstract
Cataloguing gene expression during development of the genitourinary tract will increase our understanding not only of this process but also of congenital defects and disease affecting this organ system. We have developed a high-resolution ontology with which to describe the subcompartments of the developing murine genitourinary tract. This ontology incorporates what can be defined histologically and begins to encompass other structures and cell types already identified at the molecular level. The ontology is being used to annotate in situ hybridisation data generated as part of the Genitourinary Development Molecular Anatomy Project (GUDMAP), a publicly available data resource on gene and protein expression during genitourinary development. The GUDMAP ontology encompasses Theiler stage (TS) 17 to 27 of development as well as the sexually mature adult. It has been written as a partonomic, text-based, hierarchical ontology that, for the embryological stages, has been developed as a high-resolution expansion of the existing Edinburgh Mouse Atlas Project (EMAP) ontology. It also includes group terms for well-characterised structural and/or functional units comprising several sub-structures, such as the nephron and juxtaglomerular complex. Each term has been assigned a unique identification number. Synonyms have been used to improve the success of query searching and maintain wherever possible existing EMAP terms relating to this organ system. We describe here the principles and structure of the ontology and provide representative diagrammatic, histological, and whole mount and section RNA in situ hybridisation images to clarify the terms used within the ontology. Visual examples of how terms appear in different specimen types are also provided.
Keywords: genitourinary development, renal development, kidney development, urinary development, reproductive development, kidney, gonad, bladder, ureter, urethra, genital tubercle, ovary, testis, congenital defects, gene expression, ontology, annotation, database, anatomy, atlas of development, partonomic ontology, RNA in situ hybridisation
1. Results and Discussion
1.1 Development and disorders of the genitourinary tract
The vertebrate genitourinary tract comprises the urinary system (kidneys, ureters, bladder, urethra) and the reproductive system (external genitalia, prostate, gonads, associated ducts (fallopian tubes, vas deferens and epididymis) and uterus). The genitourinary tract arises from both mesodermal and endodermal components, but the intersection of these components occurs early in development and ultimately they are so closely linked that congenital anomalies or damage in one part frequently affects the other. The kidneys, ureters and internal genitalia (gonads, uterus, oviducts, epididymis and vas deferens) arise from the intermediate mesoderm. The urethra and bladder form initially as part of the endodermal cloaca. The urorectal septum divides the cloaca to form the urogenital sinus on the ventral side and the hindgut on the dorsal side. The external genitalia form from the genital tubercle that, like the gonads, is initially undifferentiated. At eight weeks of human gestation, sex determination occurs and, four weeks later, hormones produced from the gonads direct the development either of clitoris and labia or penis and scrotum.
The European Surveillance of Congenital Anomalies, Eurocat, reported that in Europe between 1996 and 2001, congenital anomalies of the internal genitourinary tract (excluding external genitalia) represented the third most frequent of all human birth defects, occurring in approximately 28/10,000 live births (Eurocat Annual Report, 2003). Only cardiovascular (61/10,000) and limb (37/10,000) defects were more prevalent. Genitourinary defects can vary from severe (bladder exstrophy, posterior urethral valves, persistent cloaca, cystic kidney disease, hydronephrosis, streak gonads, diphallia) to more moderate (hypospadias, horseshoe kidney, micropenis, cryptorchidism, unilateral renal agenesis). Some of these defects (e.g. horseshoe kidney, unilateral renal agenesis) do not require surgical intervention and may not even be diagnosed (Weizer, 2002), while others may require extensive reconstructive surgery. The latter include bladder reconstruction for persistent cloaca, organ transplantation for cystic kidney disease and renal dysgenesis, removal of streak gonads and Müllerian duct remnants for XY pseudohermaphroditism and penile reconstruction for hypospadias and diphallia (Gyftopoulos, 2002; Warne, 2002). The developmental relationship between the urinary and reproductive systems is strongly reflected in the combination of observed defects.
Postnatal-onset disease of the urogenital tract is also a major problem. Cancer can arise in all parts of the urogenital tract with prostatic cancer showing the highest prevalence (http://info.cancerresearchuk.org/cancerstats/incidence/prevalence/). Some cancers of the urogenital tract (gonadoblastoma, Wilms’ tumour) represent a persistence of the embryonic state. Chronic renal disease, due to polycystic kidney disease, cardiovascular disease, injury, infection, glomerulonephritis or diabetes, results in end stage renal disease (ESRD). The link between development and renal disease comes from evidence that the number of nephrons present in each kidney is inversely correlated with the chance of chronic renal disease later in life (Hoy et al, 2005). Conversely, adult onset renal disease and other disorders of the genitourinary tract show reactivation of molecules key to the normal development of the genitourinary tract.
1.2 The Genitourinary Development Murine Atlas Project
All these observations highlight the need to comprehensively understand the molecular basis of genitourinary development. While this understanding is not attainable in humans, the need has catalysed the creation of the Genitourinary Development Molecular Anatomy Project (GUDMAP). GUDMAP aims to chronicle the expression of genes, both temporally and spatially, during the development and maturation of the murine genitourinary tract and to create tools for the scientific community to examine the biological function of these genes. The creation of an atlas of gene expression will serve as a reference point in analyses of lineage, cell fate and disease within this organ system. Gene expression data to be held in the atlas will include microarray, RNA in situ hybridisation and immunohistochemical information. Well-characterized markers of structures within the genitourinary tract will also help to standardize mutant analyses and enable more subtle phenotypes to be recognised. Data generated within this project are being made freely publicly available via a central database (http://www.gudmap.org). The need for these data to be annotated accurately was a driving force in the development of a text-based anatomical ontology of the genitourinary tract. By developing a defined ontology, this will enable the efficient computational linking of different types of molecular analyses (e.g microarray and in situ hybridisation) adding to our understanding of development at a systems biology level.
The Edinburgh Mouse Atlas Project (EMAP; http://genex.hgu.mrc.ac.uk/intro.html) developed a 3D digital atlas of mouse embryonic development based on the definitive texts of Theiler (1989) and Kaufman (1994) in which the models contain defined anatomical domains linked to a stage-by-stage text-based hierarchical ontology of anatomical nomenclature. A descriptive anatomical ontology was developed to define these domains. This combination of three dimensional models and text-based anatomical nomenclature has been exploited in the Edinburgh Mouse Atlas of Gene Expression (EMAGE; http://genex.hgu.mrc.ac.uk/Emage/database/emageIntro.html) (Baldock et al, 2003) in which gene expressions are painted onto the models. The EMAP anatomical ontology has been adopted by the Gene Expression Database (GXD) at the Jackson Laboratory (http://www.informatics.jax.org/mgihome/GXD/aboutGXD.shtml) and has provided an invaluable resource for the developmental biology community both for the teaching of developmental anatomy and the broad description of gene and protein expression patterns. However, the complexity of the developing mouse is such that the degree of detail provided within the EMAP ontology is not sufficient to provide a precise description of a gene or protein expression pattern at high resolution within specific tissues or organs after mid-gestation. However, the ontology is amenable to modification such that a specific organ system can become more highly annotated without disrupting the overall structure of the ontology. The objective of this study was to create an anatomical nomenclature for the developing murine genitourinary tract that could be inserted into the existing ontological nomenclature of EMAP.
1.3 Structure and principles of the murine genitourinary ontology
This genitourinary anatomical ontology extends from Theiler stage (TS) 17 (approximately 10.5dpc) to TS27 (approximately 19.5dpc to postnatal day 3). The ontology also describes the sexually mature adult mouse (defined here as TS28sm). The ontology for both the developing and postnatal genitourinary tract incorporated existing EMAP terms (where possible), published literature and examination of the developing genitourinary tract in consultation with international experts. The ontology for the murine adult kidney was developed as an extension of the EMAP embryonic anatomy with reference also to the GXD Dictionary of Adult Mouse Anatomy (http://www.informatics.jax.org/searches/anatdict_form.shtml), the Foundational Model of Anatomy (FMA, http://sig.biostr.washington.edu/projects/fm/FME/index.html) and publications describing the anatomy of the mouse and human kidney (listed throughout the text).
The structure of the ontology is the same as used for the EMAP anatomy ontology (Bard et al, 1998; Burger et al, 2004a). By analogy with a physical dissection, the terms within this ontology encompass a set of fine subdivisions of genitourinary anatomy that are non-overlapping and represent the entire material of the system. This satisfies the basic requirements that a nomenclature for annotation should be unambiguous and complete. This set represents those parts of the genitourinary system that could be resolved and defined anatomically in histochemical staining procedures (e.g. toluidine blue or haematoxylin and eosin) paraffin sections. Some well-known cell types or structures previously characterised using gene or protein expression were also included. It was necessary, in a few instances, to distinguish structures that have not yet been named. These are the parts that are left over after segregating well-defined, named parts. In these cases we have used the term ‘rest of …’; for example, ‘rest of renal interstitium’. The description of a cell type (e.g. podocyte) was generally achieved using a more generic term for the anatomically visible grouping of these cells (e.g. visceral epithelium; syn: podocyte layer). While not all cell types are currently discernable via histology or by specific gene expression, this does not preclude the subsequent insertion of specific cell types at a later date as the current working version is open-ended. While every attempt was made to integrate all existing terms from within EMAP, some were removed or renamed. Where possible, synonyms (‘syn’) were used to ensure the continued use of existing terms. This also allowed the incorporation of multiple names for the same structure where there is discordance in the anatomical terminology.
Having defined the components, the ontology organises this set of structures as a 2-dimensional, hierarchical tree, naming progressively larger parts (e.g. renal vesicle, renal cortex, metanephros) as the tree moves from branches to trunk. Thus at Theiler Stage (TS) 20, ‘ureteric tip’ and ‘ureteric trunk’ are parts of the larger term ‘primitive collecting duct’. A positive gene expression result for the ureteric tip implies expression in the primitive collecting duct. Conversely, a negative result in the primitive collecting duct implies a negative result in the ureteric tip. Thus expression is inherited upwards and lack of expression is inherited downwards in the anatomical tree.
It is not possible to represent all commonly named structures in any single primary tree. Consider, for example, the nephron, renal cortex and renal medulla. One would wish to have these terms included in the ontology, yet a single partonomic arrangement must either separate the cortex from the medulla (thus dividing the nephron) or dissect out the nephron which extends across cortex and medulla. To address this problem we have supplemented the primary tree with alternative views in order to represent the desired additional structures. We have called these views ‘groups’. Thus, for example, the primary tree separates cortex and medulla; secondarily, we have grouped together the cortical and medullary components of the excretory unit to form the ‘nephron group’. With the inclusion of groups, the hierarchy can be represented as a directed acyclic graph in which anatomical components can be a part of more than one structure. In practice, we have used these terms to group together components in different ways in order to facilitate annotation of gene expression.
Because annotations are made on individual organisms, each at a particular stage of development, we have given a separate, stage-specific unique EMAP identifier (EMAP:ID) to each anatomical term. These IDs are then used to annotate gene expression patterns in a stage-specific way. We have not captured the developmental relations between different, named structures (e.g. renal vesicle is a precursor of the comma-shaped body). Hence relationships between a given terms and its component terms does not imply lineage. In addition, the structures represented at each developmental stage are those present at any time during that stage, but not necessarily throughout the entire stage interval. Hence when using any anatomical term to annotate, for example, a gene expression pattern, the annotation will refer to a moment in time and, because of the dynamic nature of gene expression, will not necessarily pertain across the entire stage.
1.4 The GUDMAP anatomy and gene expression databases
The complete ontology for the genitourinary tract has been incorporated into the GUDMAP anatomy database (http://www.gudmap.org). That database interface presents different views of the ontology according to the requirements of the user. The ontology will also be used in the EuReGene Renal Genomics Project (EuReGene) (http://www.euregene.org) and will be used to update the EMAP ontology used by the GXD (http://www.informatics.jax.org/mgihome/GXD/aboutGXD.shtml). The publicly accessible, GUDMAP gene expression database uses the ontology to annotate, query and display results from RNA in situ hybridisation and microarray gene expression experiments. The database can be queried using a tree view of the ontology from http://genex.hgu.mrc.ac.uk/gudmap/gudmapdbquery?query=queryentrypage. Results are returned as a list of genes expressed in the selected anatomical structures. Query searches of the in situ hybridisation database can also be performed using the appropriate EMAP identification number for the structure. This will generate a list of entries for which this structure has been annotated as present. In any given entry, the expression pattern of the relevant gene is displayed in the context of a tree display of the anatomy ontology, indicating structures with expression present, expression possible, expression not present and expression not examined. We also plan to make the ontology accessible in standard format via the OBO site (http://obo.sourceforge.net).
1.5 Use of partonomic ontologies for annotation to varying levels of resolution
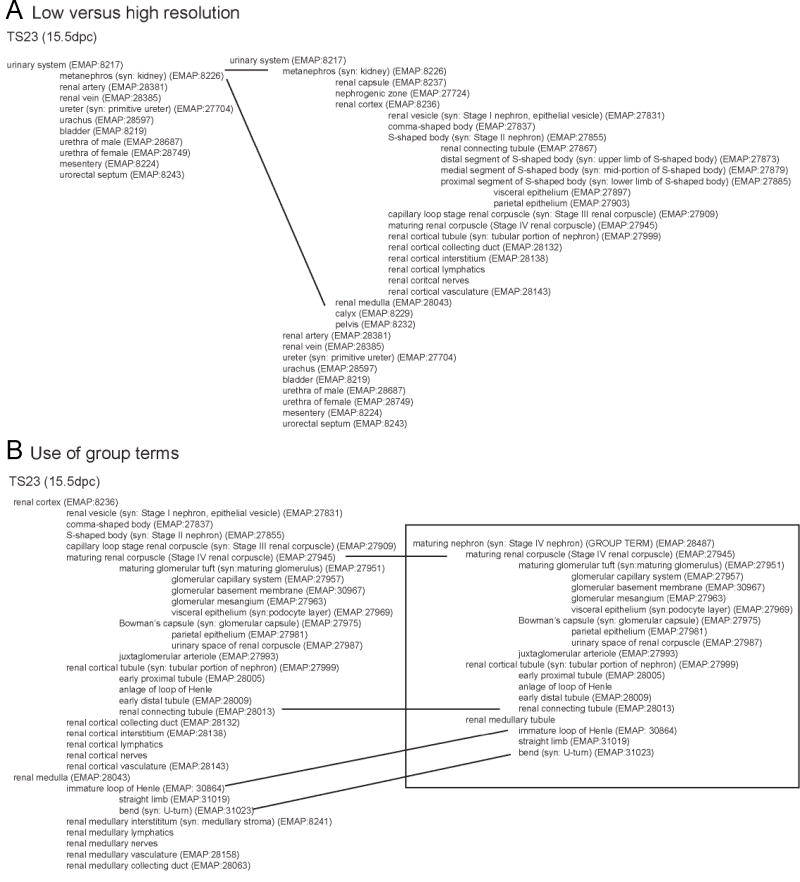
It was important that the ontology could be used to annotate patterns of gene or protein expression on both low (whole mount) and high-resolution (sectioned) material. This is particularly relevant when describing an organ system small enough to have whole mount preparations of tissues past midgestation that exhibit quite complex structures. Because it is not necessary to annotate to the tips of the branches of this partonomic tree, an annotator can annotate a pattern of expression at high or low resolution using the same ontology. Indeed, some regions can be annotated at high resolution and others at low resolution depending upon the confidence with which any given set of structures appear to show expression. It has also been made applicable to both whole mount and sectioned material via the creation of group terms, which are alternate arrangements of parent / children relationships that may be more suited to the description of a structure in one type of material versus another. In Figure 1, an example tree showing the portion of the ontology dealing with the genitourinary tract at TS23 is displayed at a variety of levels of resolution to illustrate how it can be used for either whole mount or section material.
Figure 1. Alternate views of the ontology demonstrating high and low resolution annotation and group terms.
a) Tree view of ontology for TS23 demonstrating the utility of the ontology for both high and low resolution annotation. b) Tree view at TS23 demonstrating the subcomponents of the maturing renal corpuscle (renal corpuscle of Stage IV nephron) in the cortex and the subdivision of the renal tubules between renal cortex and renal medulla (left) compared to the group term for maturing nephron (Stage IV nephron) (right). The remainder of the ontology is fully documented at http://www.gudmap.org/Resources/Ontologies.html.
The complete ontology covering all stages from TS17 to TS27 and adult (TS28sm) is presented as Supplementary data (Supplementary Tables 1–5) and can be viewed at http://www.gudmap.org/Resources/Ontologies.html. A tutorial describing the important events in the development of the murine urogenital tract can also be found at http://www.gudmap.org/About/Tutorial/. The following descriptions detail how the components of the genitourinary tract have been subdivided at each timepoint. The specific EMAP identification numbers referred to in this text relate to the Theiler stage being described or the stage at which this term first appears in the ontology.
1.6 An ontology of the genitourinary tract from TS17 to TS19
Between TS17 and TS19, the ontology was created to describe the developing genitourinary tract as a whole, including the mesonephros, metanephros, gonad, cloaca, urogenital sinus and associated structures. The ontology from TS17 to TS19 is represented diagrammatically in Figure 2A. Figure 2B illustrate example gene expression patterns for some of the components assessed using whole mounts of urogenital tracts at TS17.
Figure 2. Representative images and example whole mount and section in situ expression patterns from TS17 to TS23.
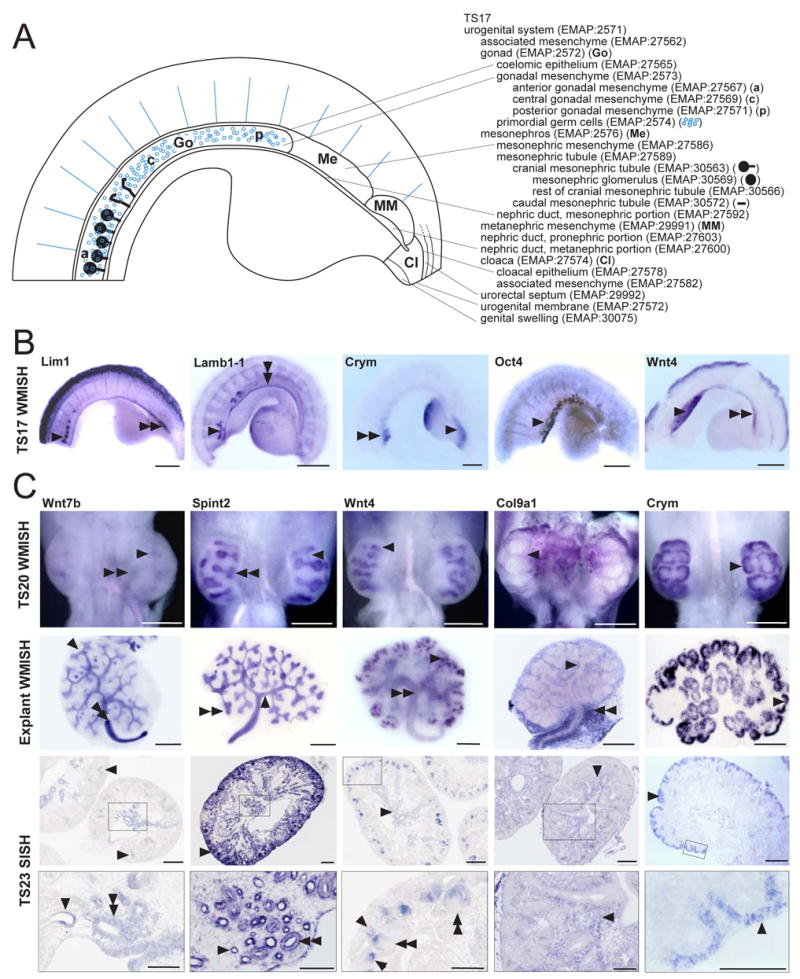
A. Diagrammatic representation of the TS17 urogenital system accompanied by the complete ontology for this stage. TS17 embryos were dissected transversely below the forelimbs and longitudinally down the midline to expose the urogenital system. The caudal end is on the right and the hindlimb is visible. B. Representative examples of whole mount RNA in situ hybridisation at TS17 showing expression of Lim1 in the nephric duct (double arrowhead, EMAP:28426) and mesonephric glomeruli (arrowhead, EMAP:30569), Lamb1-1 in the nephric duct (double arrowhead, EMAP:28426) and mesonephric tubules (arrowhead, EMAP:27589), Crym in the metanephric mesenchyme (arrow, EMAP:29991) and mesonephric tubules (double arrowhead, EMAP:27589), Oct4 in the primordial germ cells (arrow, EMAP:2574) and Wnt4 in the gonad (arrowhead, EMAP:2572), mesonephric mesenchyme (double arrowhead, EMAP:27586), mesonephric tubules (EMAP:27589) and nephric duct, mesonephric portion (EMAP:27592). Scale bar = 50μm. C. Representative examples of RNA in situ hybridisation of TS20 urinary system, TS20 metanephroi explanted and cultured for 2 days and TS23 metanephros sections. The same genes (Wnt7b, Spint2, Wnt4, Col9a1, Crym) have been compared between these different stages to demonstrate the patterns seen for genes with specific expression in distinct compartments. Two images are shown at TS23, a global view of the metanephros with a high magnification image below (the area magnified is outlined). Wnt7b: TS20 and explanted metanephroi showed Wnt7b expression in the ureteric trunk (arrowheads, EMAP:27698) and in the ureter (double arrowheads, EMAP:27701). Expression was not detected in the ureteric tips. At TS23, the global view shows Wnt7b expression in the cortical collecting ducts (arrowheads, EMAP:28132) and high magnification of the medulla shows expression in medullary collecting ducts (double arrowhead, EMAP:28063) and immature loop of Henle (arrowhead, EMAP:28173). Spint2: Spint2 was expressed in the ureteric tip (double arrowhead) and ureteric trunk (arrowhead) of TS20 and explanted metanephroi and in the ureter of explants. At TS23, Spint2 expression continued in the ureteric tip (arrowhead in global image) and cortical and medullary collecting ducts (double arrowhead in high magnification) and was also seen in cortical and medullary renal tubules, including the immature loop of Henle (arrowhead in high magnification). Wnt4: In TS20 and explanted metanephroi, Wnt4 was expressed in early nephrons (arrowheads, EMAP:29339). Later, in explants and at TS23, Wnt4 was detected in cortical and medullary collecting ducts and the cortical and medullary interstitium immediately surrounding the collecting ducts (double arrowhead in explant, arrowhead in global image TS23) and in the pelvic urothelial lining (TS23) and ureter (explant). Expression persisted in early nephrons at TS23 with expression in pretubular aggregates, renal vesicles (arrowhead in high magnification), comma-shaped bodies and S-shaped bodies (double arrowheads in high magnification). Col9a1: Col9a1 was expressed in the renal interstitium at all stages (arrowheads), including the perihilar interstitium (double arrowheads, explant). Crym: Crym was strongly and specifically expressed in the cap mesenchyme at all stages presented (arrowheads). We have previously described an ontology for explanted kidneys (Martinez et al, 2005). Scale bar = 200μm. Images and diagrams courtesy of Kylie Georgas, Bree Rumballe, Hansheng Chiu, Grant Challen, Michael Lusis and Melissa Little.
The nephric duct (syn: mesonephric duct; Wolffian duct), which extends along the length of the genitourinary tract, was partitioned into ‘nephric duct, pronephric portion’, ‘nephric duct, mesonephric portion’ and ‘nephric duct, metanephric portion’. This regional partitioning resulted from the observation that this duct runs through the mesonephros itself and hence a portion of it needs to be described as being a part of the mesonephros. This is in contrast to the metanephric portion, so named as being adjacent to the region of metanephric formation, which is never a part of the metanephros itself. This partitioning also allows a separation of the urinary and reproductive systems from TS19. The term mesonephric tubule, a part of the mesonephros, refers to the tubules growing out from the ‘nephric duct, mesonephric portion’ into the mesonephric mesenchyme. This has been subdivided into cranial and caudal mesonephric tubules. Cranial mesonephric tubules have been subdivided into mesonephric glomerulus and rest of cranial mesonephric tubule (Saino et al, 1997).
At TS17, there is no metanephros, but there is a discernable area of intermediate mesoderm caudal to the mesonephros and adjacent to a widening of the ‘nephric duct, metanephric portion’ that gives rise to the metanephros. At TS17 this is the metanephric mesenchyme. The term metanephros exists from TS18, and now includes the metanephric mesenchyme, ureteric bud, itself subdivided into the ureteric tip (syn: ureteric ampulla) and ureteric stalk (syn: ureteric trunk). From TS19, a distinction is made between the urinary system and the reproductive system with the gonad, mesonephros and genital tubercle forming the reproductive system from here on. The metanephros at TS19 remains subdivided into metanephric mesenchyme and ureteric bud (and children thereof), but metanephric mesenchyme is now separated into peripheral blastema and cap mesenchyme.
In the region of the lower urinary tract between TS17 and TS19, the urorectal septum is still in the process of dividing the cloaca to form the hindgut and urogenital sinus, a process thought to involve apoptosis (Yamada et al, 2006). At TS17, only the cloaca is included but from TS18-19, both cloaca and urogenital sinus are present. The intermediate mesoderm surrounding the genitourinary tract is referred to as the parametanephric mesenchyme (syn: associated mesenchyme) while associated mesenchyme as a part of cloaca specifically refers to that region around the cloaca. The term urogenital membrane is used, although this region is also called cloacal membrane.
The gonad (syn: gonad primordium) is annotated as indeterminate between TS17 and 19, although the expression of Sry initiates at 10.25dpc (TS17) (Koopman et al, 1990), making it possible that there will exist sexually dimorphic gene expression patterns in all parts of the genitourinary tract from this stage. The mesenchyme of the gonads from TS17-19 is divided into anterior gonadal mesenchyme (syn: rostral gonadal mesenchyme), central gonadal mesenchyme and posterior gonadal mesenchyme (syn: caudal gonadal mesenchyme). This reflects the observation of rostral-caudal differences in the expression of genes within the developing gonads, with Sry expression commencing centrally and then moving towards both poles and extinguishing from anterior to posterior (Bullejos and Koopman, 2001). Primordial germ cells are listed as clear molecular evidence of their existence at this stage comes from the expression of genes such as c-kit (Orr-Urtreger et al, 1990) and Oct4 (Scholer et al, 1989; see Figure 2). The final component of the gonad is the coelomic epithelium (syn: germinal epithelium). At TS19, the reproductive system is distinguished from the urinary system and mesonephros, gonad and genital tubercle are listed parts of the reproductive system. The paramesonephric duct is also first included as a term at this stage (Kobayashi and Behringer, 2003; Yin and Ma, 2006).
At TS17 and TS18, the paired genital swelling (syn: genital fold), which will contribute to the external genitalia is seen (Perriton et al, 2002; Yamada et al, 2003; Suzuki et al, 2004). The term genital tubercle first appears at TS19, at which time it is subdivided into the dorsal genital swelling and the ventrolateral genital swelling. The ventrolateral genital swelling gives rise to the urethral plate.
1.7 The ontology of the developing metanephros and ureter from TS20 to TS27
General subdivisions of the kidney and ureter
From TS20, the renal / urinary system is subdivided to include the metanephros (syn: kidney), nephric duct, ureter, allantois, urogenital sinus, which will give rise to the bladder and urethra, urorectal septum, urethral plate and urethral fold. The metanephros is the most complex of these structures. At TS20, this is now subdivided into the renal interstitium, cap mesenchyme, pretubular aggregate, renal vesicle, comma-shaped body, developing vasculature and primitive collecting duct. A number of subcomponents of the renal interstitium are defined, including nephrogenic interstitium, which is located at the periphery of the developing kidney, and several cell types including renal fibroblasts and resident macrophages. The latter are first present within the renal interstitium from TS20 and persist throughout kidney development, residing in the adult in close apposition to the renal tubules (our unpublished data). The first group term, early nephron (syn: early tubule), is defined as including pretubular aggregate, renal vesicle and comma-shaped body as analysis by whole mount RNA in situ hybridisation (WMISH) is unlikely to be able to distinguish the separation. The ontology from TS21 begins to subdivide the metanephros regionally, which is most useful for the annotation of sections, but includes group terms more relevant to the annotation of whole mount preparations. At TS21, these subdivisions include renal capsule and nephrogenic zone. The nephrogenic zone, which is found at the periphery of the developing metanephros, is the region within which the metanephric mesenchyme aggregates around the tips of the ureteric tree to form the nephrons. In rodents including mice, this region persists for a few days postnatally, although it is lost in humans by 36 weeks of gestation. The children of nephrogenic zone include the ureteric tip (syn: ureteric ampulla), ureteric tree terminal branch, nephrogenic zone interstitium (syn: peripheral blastema), cap mesenchyme (syn: condensed mesenchyme) and pretubular aggregate. The latter is distinct from renal vesicle by virtue of the absence of polarisation and the lack of E-cadherin expression. From TS22, the regional subdivision of the metanephros includes the renal capsule, nephrogenic zone, renal cortex, renal medulla, pelvis and renal artery and renal vein. From TS23, the calyx is also included as components of metanephros. Figure 3 provides diagrammatic and light microscopic histological examples of terms described within the TS23 developing metanephros. This stage is represented as it contains all phases of nephron development. By TS25, the medulla is subdivided into outer medulla and inner medulla (syn: papilla).
Figure 3. Representative images and example sections demonstrating the terms present within the murine TS23 metanephros.
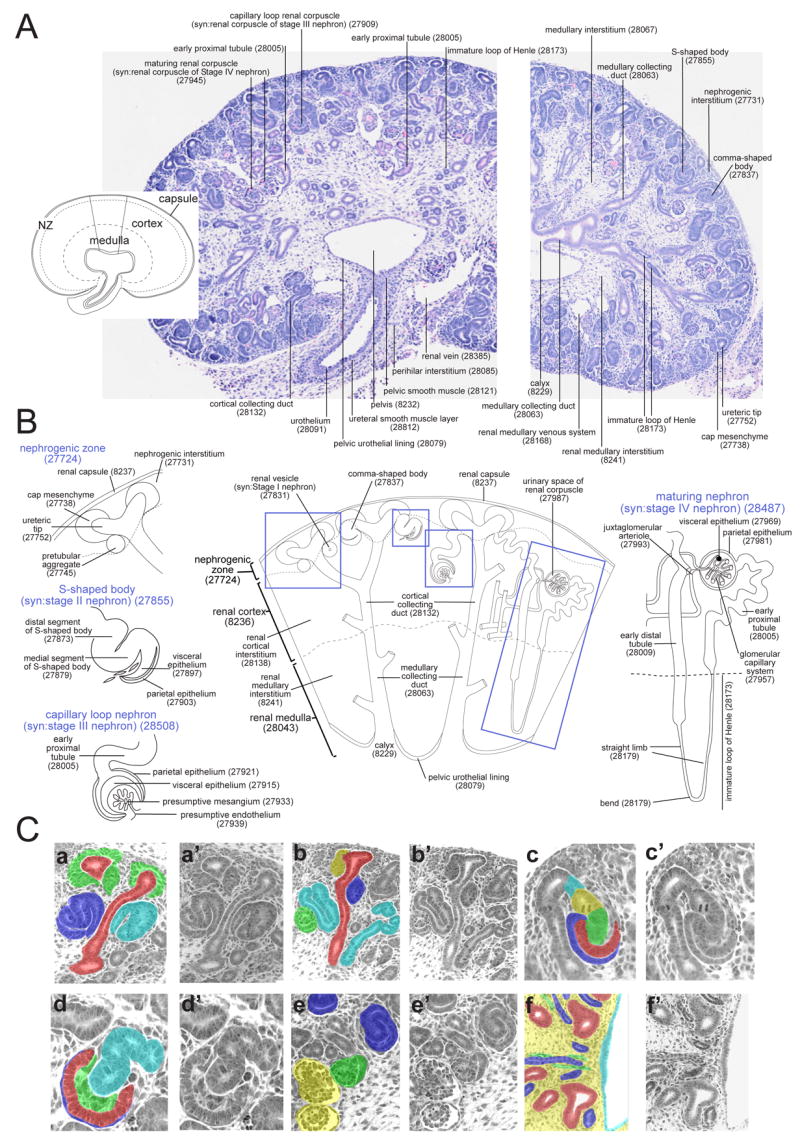
A. Diagram and histological sections of the complete murine metanephros at TS23 annotated using terms from the ontology or their corresponding EMAP identification numbers. The inset diagram (left) shows the broad regions of the metanephros defined in the ontology and indicates the wedge of metanephros enlarged in Figure 3B. The histology sections (haematoxylin and eosin) show sagittal sections through the metanephros and ureter (left) and just beyond the midline of the metanephros (right).
B. Diagrammatic representation of a wedge of the TS23 metanephros, from renal capsule to pelvis, annotated using terms from the ontology along with their corresponding EMAP numbers. Dashed boxes indicate the location of Stage I to IV nephrons that have been annotated in detail along the left and right sides of the diagram.
C. Series of matched-pair histological images from a TS23 metanephros colouring specific anatomical terms present within the ontology. a,a′) nephrogenic zone and cortex highlighting ureteric tip (EMAP:27752, red) and renal cortical collecting duct (EMAP:28132, red), cap mesenchyme (EMAP: 27738, green), comma-shaped body (EMAP: 27837, light blue) and s-shaped body (syn: Stage II nephron, EMAP:27855, dark blue). b,b′) nephrogenic zone and cortex showing the ureteric tip (EMAP:27752, red) and renal cortical collecting duct (EMAP:28132, red), pretubular aggregate (EMAP:27745, yellow), renal vesicle (syn: Stage I nephron, EMAP:27831, dark blue), capillary loop stage renal corpuscle (Stage III renal corpuscle, EMAP: 27909, green) and renal tubules (EMAP: 27999, light blue). c,c′) Segments of the s-shaped body (Stage II nephron) including the visceral epithelium (EMAP:27897, red), parietal epithelium (EMAP: 27903, dark blue), medial segment (EMAP: 27879, green), distal segment (EMAP:27873, yellow) and renal junctional tubule (EMAP:27867, light blue). d,d′) Segments of the capillary loop stage renal corpuscle (Stage III renal corpuscle) including the visceral epithelium (EMAP:27915, red), parietal epithelium (EMAP:27921, dark blue), presumptive mesangium (EMAP:27933, green) and renal tubule (EMAP:27999, light blue). e,e′) Cortex of the TS23 metanephros showing different stages of renal corpuscle development including s-shaped body (syn: Stage II nephron, EMAP:27855, dark blue), capillary loop stage (syn: Stage III, EMAP:27909, green) and maturing (syn: Stage IV, EMAP:27945, yellow) renal corpuscles. f,f′) Medulla and pelvis of the TS23 metanephros showing the renal medullary interstitium (EMAP:8241, yellow), medullary collecting ducts (EMAP:28063, red), immature loop of Henle (EMAP:28173, dark blue), renal medullary vasculature (EMAP:28158, green) and pelvic urothelial lining (EMAP:28079, light blue). Histological images courtesy of Luise Cullen-McEwen and Melissa Little. Diagrams courtesy of Kylie Georgas.
Stages of nephron induction
As the metanephros develops and nephron induction occurs, four stages of nephron development have been defined in the literature using light and electron microscopic observations (Larsson, 1975, Reeves et al, 1978, Larsson and Maunsbach, 1980, Johkura et al, 1998). An electron microscopic analysis by Larsson (1975), taking into account the degree of proximal tubule development and differentiation, noted the presence of microvilli on the proximal tubules by Stage III with a brush border present at Stage IV. This subdivision of stages does not include a stage for the comma-shaped body. The term comma-shaped body, however, is in common use and is histologically and morphologically definable. For this reason, the terms Stage I to IV nephron are listed as synonyms for renal vesicle (Stage I), S-shaped body (Stage II), capillary loop stage (Stage III) and maturing nephron (Stage IV) (see Figure 3B). In the ontology, renal vesicle, comma-shaped body and S-shaped body are present from TS20. The comma-shaped body is subdivided into an upper and lower limb and the S-shaped body into distal, medial and proximal segments to accommodate the differential regional expression of genes previously reported in these structures (Georgas et al, 2000; Marlier and Gilbert, 2004; Chen and Al-Awqati, 2005). The S-shaped body, present from TS21, is physically connected to the adjacent ureteric tip (syn: ureteric ampulla) via a renal connecting tubule (Potter, 1972). Capillary loop (Stage III) is first included at TS21 and maturing nephron (Stage IV) from TS22. An expanded view of the components present in an S-shaped body at TS23 is shown in Figure 1a. The term mature nephron is present from TS25 and it is at this stage that the group term juxtaglomerular complex is included with the components extraglomerular mesangium, macula densa and part of the afferent arteriole forming the juxtaglomerular complex. Each of these is also a subcomponent of mature renal corpuscles, distal straight tubules and afferent arterioles respectively.
Annotation of the developing renal tubules
As the analysis of expression data in either section or whole mount material will not allow a simple association of expression in a particular tubule with a given renal corpuscle, the renal tubules were described separately to the stages of the renal corpuscle. This also allowed a regional subdivision such that the immature loop of Henle occurs within the medulla rather than the cortex and the renal tubules within the cortex include early proximal tubule, anlage of loop of Henle, early distal tubule and renal connecting tubule (see Figure 1b). However, group terms were constructed for each stage of nephron such that the renal corpuscle and associated renal tubules were grouped together to create the terms developing capillary loop stage nephron (syn: Stage III nephron) and maturing nephron (syn: Stage IV nephron) (see Figure 1b). From TS25, there is also a mature nephron group term. Nakai et al (2003) in their analysis of the effect of a Brn1 deficiency in the mouse kidney, defined three stages of loop of Henle differentiation; anlage, primitive and immature loops. The former were regarded as residing within the cortex of the TS24 kidney, whilst the term immature loop of Henle was used to describe those regions within the medulla. In the ontology, we have made a cortical and medullary separation only, with the cortical loop of Henle referred to as the anlage of the loop of Henle (syn: primitive loop of Henle) and the medullary referred to as the immature loop of Henle. These terms are first described from TS22. Immature loop of Henle encompasses the terms straight limb and U-turn. Both the anlage and immature loops of Henle form components of the group term maturing nephron, whilst the capillary loop nephron (Stage III) only contains the cortical anlage of the loop of Henle. By TS25, there is a subdivision of the medulla into outer and inner medulla. The loop of Henle is then divided regionally into the loop of Henle, outer medullary portion, and loop of Henle, inner medullary portion. Loop of Henle, outer medullary portion contains the terms thin descending limb, proximal straight tubule (syn: segment 3 of the proximal tubule, syn: pars recta) and the premacula segment of distal straight tubule (syn: thick ascending limb). Loop of Henle, inner medullary portion contains thin descending limb, bend and thin ascending limb (syn: ascending thin limb).
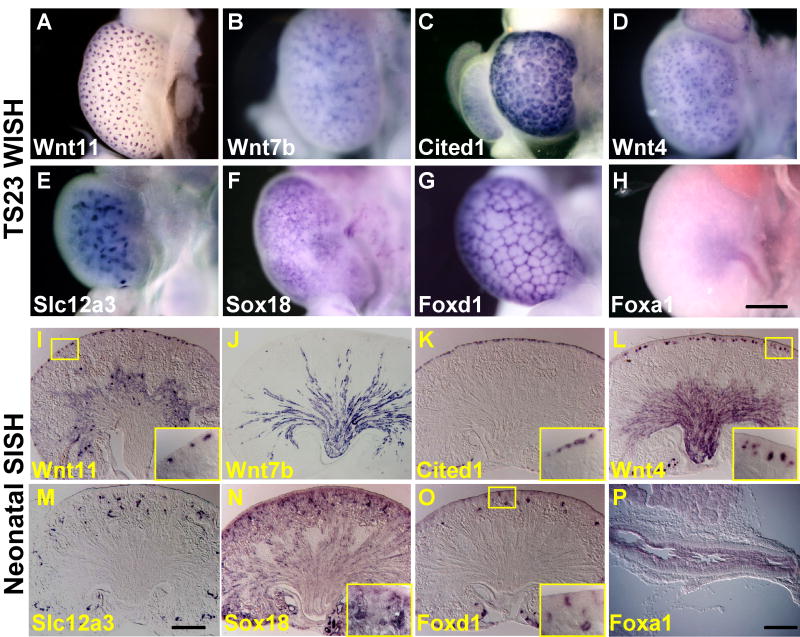
The use of group terms to accommodate low resolution annotation
The subdivision of the ontology of the metanephros from TS22 is best suited to an analysis performed by section in situ hybridisation. However, whole mount preparations of the kidney analysed at and after TS22 also need to be accommodated by the ontology. We have demonstrated how this can be addressed using TS23. An analysis of the expression of >1600 transcription factors in the TS23 kidney performed using whole mount in situ hybridisation (WISH) (Yu et al, unpublished) suggests that there are eight readily recognisable patterns in such material. These include the ureteric tip, ureteric trunk (syn: collecting duct), cap mesenchyme, early tubule, late tubule, renal interstitium, renal vasculature and ureter (Figure 4A-H). The validity of annotation of TS23 WISH into these terms is demonstrated by comparing this data with gene expression data performed using section in situ hybridisation (SISH) of neonatal (TS27) kidney sections for the same genes (see Figure 4I-P). The presence of expression in additional portions of the kidney at TS27 either indicates the onset of expression in additional compartments as development proceeds or the fact that strong expression of structures close to the surface in WISH may obscure expression in deeper structures.
Figure 4. Comparison of low resolution annotation of TS23 kidney whole mounts with the structures discernable in neonatal kidney sections.
Analysis of whole mount in situ hybridisation (WISH) of kidney and ureter at TS23 reveal 8 readily annotated patterns corresponding to the ureteric tip (A, Wnt11), ureteric trunk (B, Wnt7b), cap mesenchyme (C, Cited1), early tubule (D, Wnt4), late tubule (E, Slc12a3), renal vasculature (F, Sox18), renal interstitium (G, Foxd1) and ureter (H, Foxa1). Section in situ hybridisation of neonatal kidneys (I-P) for the same genes confirms the accuracy of these earlier annotations. Expression in deeper or more mature structures within the kidney revealed by SISH is not obvious from TS23 whole mount images either due to lack of expression of mature structures at an earlier stage or masking of deeper signal by strong superficial signals. Scale bar = 40μM. Many of the terms used to annotate whole mount material at TS23 are group terms. Images courtesy of Jing Yu.
Two of the eight patterns recognisable at TS23 by WISH, ureter and renal vasculature, relate to high-level terms in the ontology (see Figure 1a). Ureteric tip and cap mesenchyme are terms within the nephrogenic zone (see Figure 1a). The remaining terms exist as group terms within the ontology. Ureteric tip and ureteric stalk are part of group terms covering the collecting duct (derivatives of the ureteric bud). The primitive collecting duct at TS21 is a group term encompassing ureteric tip, ureteric tree terminal branch and ureteric trunk (syn: ureteric stalk). From TS22, this becomes the collecting duct, encompassing the ureteric tip, ureteric tree terminal branch and ureteric stalk, the latter being subdivided into renal cortical collecting duct and medullary collecting duct. By TS25, this also includes the inner medullary collecting duct (syn: papillary collecting duct). The pattern observed for ureteric tip and ureteric stalk in TS23 WISH is shown in Figure 4AB. Early tubule as a group term encompasses renal vesicle, comma- and S-shaped body and all subsequent children of these terms. Late tubule is a group term that encompasses Stage III and Stage IV nephron. When viewed in whole mount, an early tubule pattern is peripheral and a late tubule pattern is more central (see Figure 4DE). Interstitial elements, while appearing in most of the regional subdivisions of the metanephros, are also present as a group term, the renal interstitium. From TS22, this includes the renal cortical interstitium, renal medullary interstitium, perihilar interstitium from around the pelvis, nephrogenic zone interstitium and several interstitial elements from different stages of renal corpuscle, such as the glomerular mesangium and associated interstitium of the Stage IV renal corpuscle and presumptive mesangium of the Stage III renal corpuscle. The appearance of a renal interstitium pattern in a TS23 WISH is shown in Figure 4G. From TS21, a group term for the renal vasculature exists. The appearance of a renal vasculature pattern in a TS23 WISH is shown in Figure 4F. At TS21, this includes the renal artery, renal vein and the presumptive endothelium of the Stage III renal corpuscle. At TS22, this also includes the developing vasculature of the different regions of the metanephros, including the developing vasculature of the cortex and developing vasculature of the medulla. Each of these terms includes arterial, venous and capillary subterms. This group term also includes the glomerular capillary system and juxtaglomerular arteriole, which represent the vasculature relating to the Stage IV renal corpuscle.
Annotation of the pelvis and ureter
Annotation of the lining of the pelvis and ureter are illustrated in Figure 5A. The pelvis is lined with a pelvic urothelial lining (syn: pelvic urothelium) from TS22, below which a pelvic smooth muscle layer is annotated from TS23. Surrounding this is the perihilar interstitium, which surrounds the neck of the pelvis and the exiting ureter. The ureter, a derivative of the ureteric duct, is included as a distinct term outside of the metanephros from TS20. A number of studies have looked at the expression of genes within the developing murine ureter (Batourina et al, 2005; Mitchell et al, 2006). Sections of the developing ureter across time and at three different locations along the length of the ureter are shown in Figure 5B to illustrate the differentiation of the urothelium. At TS20, the ureter is subdivided into the epithelial layer of ureter, ureteral mesenchyme and ureteral vasculature. By TS21, the urothelium (syn: transitional epithelium) includes the superficial cellular layer and a deep cellular layer. The ureteral mesenchyme is subdivided into three layers, the subepithelial layer (syn: presumptive lamina propria), middle layer (syn: presumptive ureteral smooth muscle layer) and outer layer (syn: presumptive adventitia). By TS23, the deep cellular layer of the urothelium is further subdivided into the intermediate cell layer and the basal cell layer. The ureteral mesenchyme becomes subdivided such that the ureteric smooth muscle layer contains a smooth muscle component and an interstitium (syn: Cajal cell). A section of a TS23 ureter is illustrated in Figure 5C showing each of the subcomponents at this timepoint and an example RNA in situ hybridisation for the myosin, heavy polypeptide 11 (Myh11) gene from the pelvis to the distal end of the ureter is shown in Figure 5D. By TS25, the term ureteral mesenchyme is removed with the subcomponents becoming the lamina propria, ureteral smooth muscle and adventitia.
Figure 5. Representative images, histological sections and example RNA in situ hybridisation expression patterns demonstrating structures within the embryonic and adult pelvis and ureter.
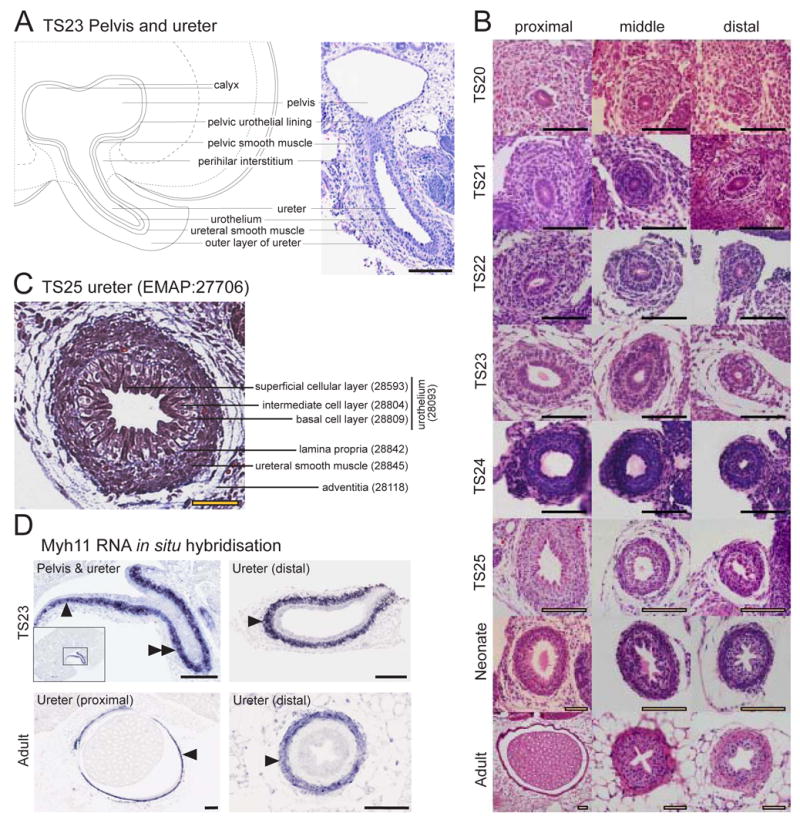
A. Low resolution diagrammatic representation (left) and histological section (right, haematoxylin and eosin) of a TS23 metanephros identifying structures around the pelvis and pelvic-ureter junction. Scale bar = 100μm. B. Temporal panel of transverse sections of the ureter at differing distances from the metanephros (proximal, middle and distal) demonstrating the histology of the developing ureter from TS20 to adult (haematoxylin and eosin). Scale bar = 100μm. C. Annotated section through a TS25 ureter middle distance between the metanephros and bladder demonstrating the cellular layers (Massons Trichrome, which stains the lamina propria blue). Scale bar = 50μm. D. Myh11 RNA in situ hybridisation of TS23 and adult ureter. TS23 Pelvis & ureter: Myh11 showed specific expression in the pelvic smooth muscle (single arrowhead, EMAP:28121) and ureteral smooth muscle layer of the ureter (double arrowhead, EMAP:28812). Inset image shows the entire metanephros section and outlines the region enlarged in the main image. TS23 Ureter (distal): Myh11 expression in the ureteral smooth muscle layer (single arrowhead, EMAP:28812) of the ureter located close to the primitive bladder. Adult Ureter (proximal): Myh11 expression in the ureteral smooth muscle layer of the ureter (arrowhead, EMAP:29488) located close to the metanephros. Adult Ureter (distal): Myh11 expression in the ureteral smooth muscle layer of the ureter (arrowhead, EMAP:29488) located close to the bladder. Scale bar = 200μm. Images courtesy of Eleanor Mitchell, Kylie Georgas, Hansheng Chiu and Bree Rumballe. Line diagram courtesy of Kylie Georgas.
1.8 The ontology of the developing lower urinary tract from TS20 to TS26
The lower urinary tract includes the derivatives of the urogenital sinus, which include the bladder and urethra, together with their subcompartments. At TS20, the urogenital sinus is subdivided into the primitive bladder (syn: cranial urogenital sinus) and rest of urogenital sinus, which will ultimately give rise to the pelvic portion of the urethra. The other components of the lower urinary tract at this time are the urorectal septum, which separates the urogenital sinus from the hindgut, the urogenital membrane and the urethral plate. The allantois, a term already existing in EMAP, is also listed in this ontology as it is contiguous with the urogenital sinus and degenerates to form the urachus, a ligament attached to the bladder. The term urachus is present from TS22.
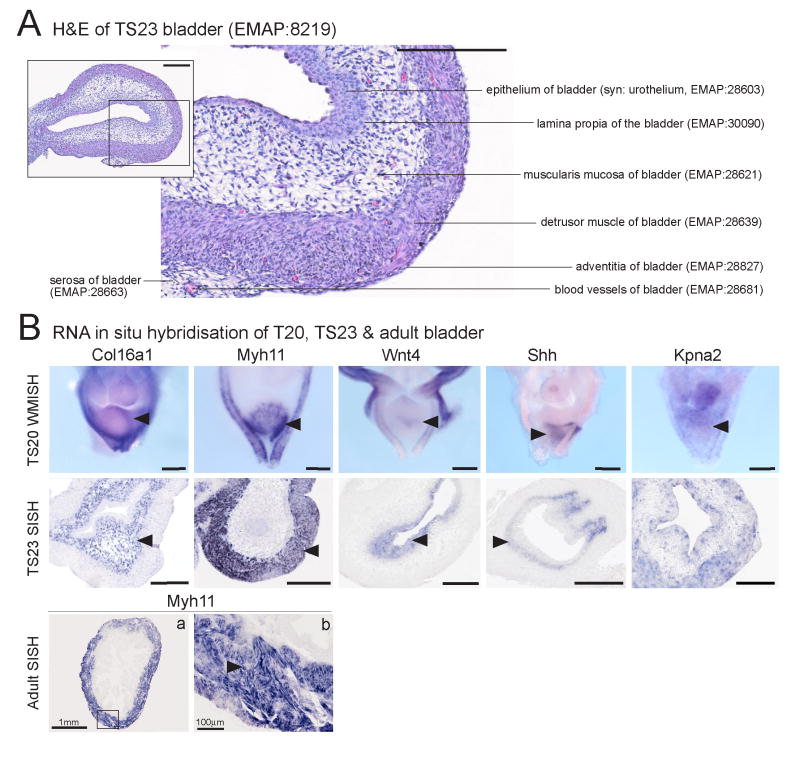
Annotation of the developing bladder
Figure 6A shows a representative section of the TS23 bladder highlighting the various layers of the bladder wall. Figure 6B shows example RNA in situ hybridisation results for TS20 whole mounts and both TS23 and adult sections of the bladder. At TS21, the term primitive bladder appears and is subdivided into the epithelium of the bladder and the mesenchyme of the bladder. At TS22, the bladder becomes subdivided into the epithelium of the bladder (syn: urothelium), lamina propria of bladder, muscularis mucosa of bladder, detrusor muscle of bladder, serosa (syn: adventitia) of the bladder and blood vessels of bladder. Each of these layers is in turn subdivided into three regional compartments; the neck, the fundus and the trigone. The trigone, as it name suggests, is a triangular region of the bladder extending between the internal orifices of the ureters and the urethra. The common nephric duct had been proposed to form the trigone as it integrated into the bladder during development, making the trigone a derivative of the mesoderm with the rest of the bladder being endodermal in origin. This has recently been challenged in the mouse when it was revealed that transposition of the ureters into the bladder involves apoptosis of the common nephric duct (Batourina et al, 2005). The fundus refers to the main body of the bladder and the neck refers to the base of the bladder from which the urethra exits.
Figure 6. Representative images and example expression patterns demonstrating structures within the embryonic and adult bladder.
(A) Histological section of the TS23 bladder identifying terms present in the ontology at this stage and their corresponding EMAP identification numbers (haematoxylin and eosin). Scale bar in (A) = 200μm. (B) RNA in-situ hybridisation of TS20 whole mount and TS23 transverse sections of the bladder for five genes and low and high magnification transverse sections of the adult bladder for Myh11. Col16a1 (Procollagen, type XVI, alpha 1) expression was seen in the muscularis mucosa of the bladder (arrowheads TS23 EMAP:28621). Expression of Myh11 was seen in the detrusor muscle of the bladder (arrowheads, TS23 EMAP:28639). Wnt4 expression was seen in the epithelium of the bladder (arrowheads, syn: urothelium; TS20 EMAP:30874; TS23 EMAP:28603). Shh was also expressed in the epithelium of the bladder (arrowheads). Kpna2 expression was seen in a scattered pattern of single cells in all layers of the bladder (arrowhead in TS20). Myh11 expression in the adult bladder: low magnification (a) and high magnification (b) images show strong expression in the detrusor muscle of the bladder (arrowhead in B) Boxed area in (a) outlines the region enlarged in (b) Myh11 was also expressed in the vasculature of the embryonic and adult bladder. Scale bar in TS20 = 50 μm, TS23 = 200 μm. Images courtesy of Kylie Georgas, Bree Rumballe and Hansheng Chiu.
Annotation of the developing urethra
At TS21, the urethra first appears as a component of urogenital sinus and is subdivided into pelvic urethra, caudal urethra, urethral plate and urethral fold. The latter represents the region that will form a groove that ultimately closes to form the penile urethra. At TS22, the urethra is subdivided into the pelvic and phallic urethra. The former is composed of an epithelial, mesenchymal and muscle layer. The phallic urethra is composed of the urethral plate and the urethral fold. The urethra is not included in the reproductive portion of the ontology but the urethra is clearly the point of direct intersection between these two parts of the genitourinary tract. From TS23, therefore, there is a distinction made between male and female urethra, although both continue to include pelvic and phallic urethra. From TS25, the phallic urethra of male is given the synonym penile urethra. The terms urethral fold and plate are replaced from this time by epithelial, mesenchymal and muscular layers of the phallic urethra.
1.9 The ontology of the developing reproductive tract from TS20 to TS26
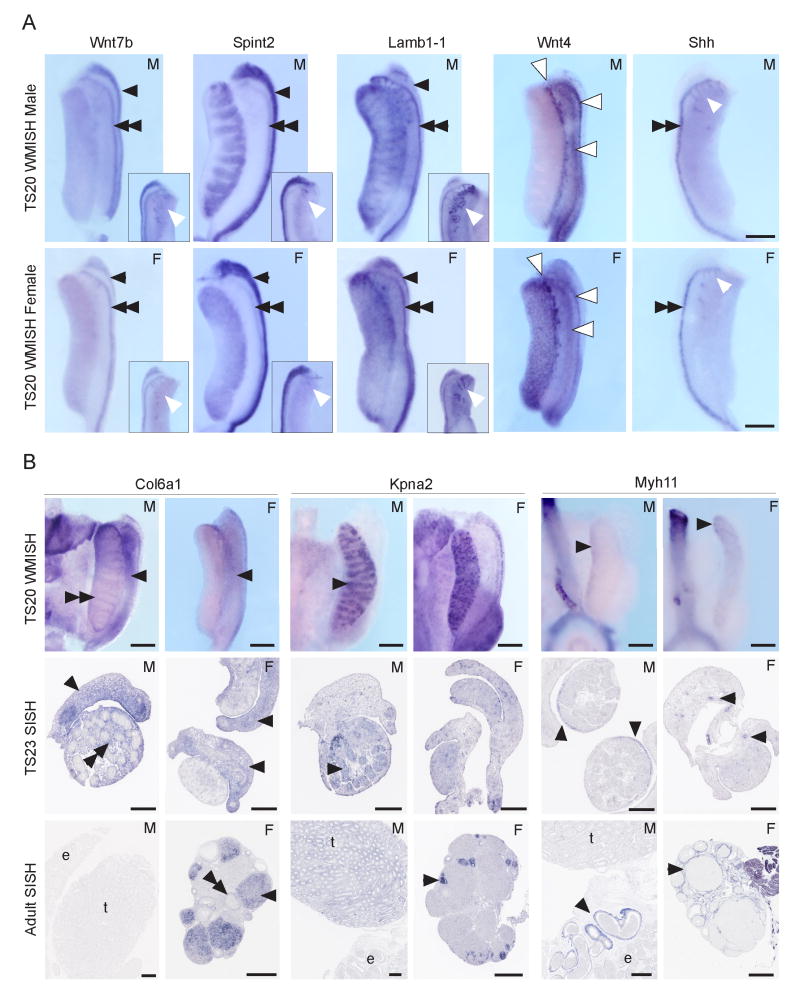
The reproductive system from TS20 onwards is subdivided into male and female as the sex of a murine gonad can be readily distinguished from this time due to the formation of the primary sex cords within the testis. Again, gradients of gene expression can be seen in the developing gonads. Meiotic genes such as Stra8 and Scp3 are expressed in an anterior to posterior (rostral to caudal) wave in the developing ovary from around TS21 (Menke et al, 2003; Bullejos and Koopman, 2004). Figure 7A illustrates some examples of sexually dimorphic patterns of expression within the male and female reproductive systems at TS20 in whole mounts of gonads. Figure 7B demonstrates the expression of specific subcompartments in both sexes across time from TS20 to adult.
Figure 7. Example gene expression patterns of the developing murine reproductive system demonstrating terms within the ontology.
A. Example whole mount in situ hybridisation of the TS20 reproductive system demonstrating the expression of 5 genes in both male (M) and female (F) reproductive systems. Wnt7b, Spint2, Lamb1-1 and Wnt4 are ventral views of the gonad-mesonephros. The dorsal view of Shh is shown to visualise the mesonephric tubules. Inset images in Wnt7b, Spint2 and Lamb1-1 are dorsal views of the rostral end of the samples to visualise the mesonephric tubules. Mesonephros: Black double arrowheads indicate expression seen in the nephric duct, mesonephric portion in both sexes, for Wnt7b, Spint2, Lamb1-1 and Shh (syn: Wolffian duct; EMAP:28925 in F; EMAP:29154 in M). Black single arrowheads indicate expression seen in the paramesonephric duct in both sexes for Wnt7b, Spint2 and Lamb1-1 (syn: Müllerian duct; EMAP:28938 in F; EMAP:29159 in M). White arrowheads indicate expression in the mesonephric tubules for Wnt7b, Spint2, Lamb1-1 and Shh in both sexes (EMAP:38920 in F; EMAP:29149 in M). Expression in the mesonephric mesenchyme was seen for Lamb1-1 and Wnt4 (EMAP:28915 in female, EMAP:29144 in male). The white outlined arrowheads in Wnt4 M and F indicate the strongest Wnt4 signal seen in the mesonephric mesenchyme, at the junction where it contacts the gonad in both sexes and in two lines of mesonephros running medial to and parallel with both the nephric duct and paramesonephric duct. Sectioning of the hybridised whole mount tissue confirmed that the Wnt4 expression was restricted to the mesonephric mesenchyme immediately surrounding the nephric duct and not in the duct epithelium. Gonad: In the male gonads, Spint2 was expressed in the primary sex cords of the testis (EMAP:29099) and Lamb1-1 in the interstitium of the testis (EMAP:29075). In the female gonads, Spint2, Lamb1-1 and Wnt4 showed expression in the ovary (EMAP:28876). Scale bar=25μm. B. Panel B shows the temporal gene expression analyses of three genes in both sexes. These are presented as TS20 whole mount, TS23 section and adult section in situ hybridisation patterns. At TS20, Col6a1 (Procollagen, type VI, alpha 1) was detected in the mesonephric mesenchyme (arrowheads, EMAP:29144 in M; EMAP:28915 in F), nephric duct (syn: Wolffian duct; EMAP:28925 in F; EMAP:29154 in M) and paramesonephric duct (syn: Müllerian duct; EMAP:28938 in F; EMAP:29159 in M) of both sexes, the interstitium of the testis (double arrowhead, EMAP:29075) and the mesenchymal stroma of the ovary (EMAP:28881). At TS23, expression persisted in the mesonephric mesenchyme of both sexes (arrowheads, EMAP:28918 in F, EMAP:29147 in M), the interstitium of the testis (double arrowhead, EMAP:29078) and both the cortical (EMAP:29010) and medullary (EMAP:29015) components of the mesenchymal stroma of the ovary. In the adult female ovary, Col6a1 (Procollagen, type VI, alpha 1) expression was seen in the corpora lutea (arrowhead) and theca cells of pre-antral and antral follicles (double arrowhead). No expression was seen in the adult male testis (t) or epididymis (e). Kpna2 was detected in a spotted pattern in all embryonic tissue types, with strongest signal detected in the primary sex cord of the testis at TS20 (arrowhead, EMAP:29099), which was maintained in the seminiferous cords of the TS23 and adult testes (arrowhead, EMAP:29230 in TS23). No Kpna2 expression was detected in the adult epididymis (e). The ovary showed Kpna2 expression at all stages (EMAP:28876: at TS20 and EMAP:8251 at TS23). In the adult ovary, strongest Kpna2 signal was detected in the granulosa cells of all follicle states and weaker signal in the oocytes of follicles (arrowhead). Myh11, a marker of smooth muscle cells, was strongly expressed in the developing aorta and common iliac arteries adjacent to the TS20 male and female reproductive tracts. Expression was also detected in the developing vasculature of the testis and ovary at TS20 and TS23 (arrowheads; EMAP:29115 (TS20) EMAP:29118 (TS23) in M; EMAP:28889 (TS20) EMAP:28892 (TS23) in F). Expression was seen in the coelomic vessel (EMAP:29123) of the testis at TS20 (arrowhead). In the adult, Myh11 expression was detected in the vasculature of the testis, epididymis, vas deferens in the male and ovary and oviduct in the female. Adult expression was also seen in the peritubular myoid cells of the seminiferous cords, the muscle layer of the epididymis (e, arrowhead) and the muscle layer of the ductus deferens (not shown) in the male and in the theca cells surrounding the follicles of the ovary (arrowhead) and in the circular and longitudinal muscle of the oviduct (not shown) in the female. Scale bar TS20=25μm, TS23=200μm, adult=500μm. Images courtesy of Kylie Georgas, Hansheng Chiu and Bree Rumballe.
Annotation of the mesonephros, paramesonephric and nephric ducts
Because of the close association between the mesonephros and the developing gonad, and literature suggesting a migration of cells from the mesonephros into the male gonad (Martineau et al, 1997; Capel et al, 1999), the mesonephros has been described as a part of the reproductive system in the ontology. Hence, mesonephros is a component within both male and female reproductive systems. At TS20, the mesonephros in the reproductive tract of both sexes consists of coelomic epithelium of the mesonephros, mesonephric mesenchyme, mesonephric tubule, ‘nephric duct, mesonephric portion’ (syn: Wolffian duct) and paramesonephric duct, mesonephric portion. At TS22 in the male, the paramesonephric duct is replaced with the term degenerating paramesonephric duct, mesonephric portion and degenerating paramesonephric duct, rest of (syn: degenerating Müllerian duct). In the female at the same stage, the mesonephric duct and tubules becomes degenerating mesonephric portion of nephric duct and degenerating mesonephric tubule. The mesonephros is present until TS24 when it is regarded as having degenerated.
Annotation of the gonads
The testis at TS20 is subdivided into coelomic epithelium (syn:germinal epithelium), interstitium of the testis, primary sex cord and developing vasculature of the testis. The components of the interstitium include the fetal Leydig cell and the rest of interstitium of testis. The components of the primary sex cord include the germ cell of the testis, Sertoli cell and peritubular myoid cell. The developing vasculature of the testis includes the coelomic vessel and the interstitial vessel. The mesorchium, a fold of the peritoneum forming a mesenteric connection between the testis and dorsal body wall, is listed outside of the testis. From TS21, the rete testis is included in testis. The rete testis refers to the point at which the seminiferous tubules coalesce and exit the testis. From TS23, the term primary sex cord is replaced with seminiferous cord. The term mediastinum testis, the connective tissue surrounding the rete testis, appears as a term within testis. From TS24, the tunica albuginea and tunica vaginalis testis are included as terms within testis. The tunica albuginea is the tough fibrous layer surrounding the testes. The tunica vaginalis testis is a sac of serous tissue covering the testis and the epididymis within which the testis can move about. This covers the tunica albuginea. The suspensory ligament of testis attaches to the rostral pole of the testis and will be critical in the descent of the testes through the inguinal canal to reach the scrotum.
The ovary at TS20 consists of the surface epithelium (syn: coelomic epithelium), mesenchymal stroma of the ovary, germ cell of the ovary (syn:oogonia; syn: premeiotic germ cell), developing vasculature of the ovary, and rete ovarii. The mesovarium lies outside of the ovary. At TS21, germ cell of ovary is subdivided into meiotic germ cell (oocyte) and premeiotic germ cell (oogonia) as germ cells begin to enter meiosis from 13.5dpc (Pepling, 2006). At TS23, the suspensory ligament of the ovary is included. At TS24, the cystic vesicular appendage is included. By TS25, the germ cells are regarded as having reached meiosis (Menke et al, 2003; Bullejos and Koopman, 2004) and the term oogonia disappears. The only term remaining to describe the female germ cell is meiotic oocytes. From TS25, the group term ovigerous cord is present and includes the germ cells of the ovary and the pregranulosa cells. From birth, the ovigerous cords begin to break to form primordial follicles comprising the primary oocyte and follicle cell (syn: granulosa cell). The term primordial follicle appears at TS27. The mesenchymal stroma of the ovary is divided regionally into the cortical and medullary components from TS22. From TS24, the cortical mesenchymal stroma of the ovary is further divided into pre-granulosa cell and rest of terms.
Annotation of associated structures of the reproductive tract
Aside from the testis, the male reproductive tract includes the epididymis and ductus deferens from TS23, the ejaculatory and seminal vesicle from TS24 and the male accessory gland, divided into bulbourethral gland (syn: Cowper’s gland) and prostate, from TS26. The epididymis at TS23 is composed of a surface epithelium, muscle layer, outer layer and developing vasculature. From TS24, this structure is separated into a caput, cauda and corpus epididymis depending upon the position along the length of this tubular structure. Each of these terms is further divided into component layers (epithelium, muscle layer, adventitia and developing vasculature). As section analysis is likely at this timepoint, in which the relative location along the length of the epididymis may be unclear, group terms have been created for each layer such that the location along the epididymis may or may not be annotated. For example, the group term muscle layer of epididymis may be annotated as such or further refined as muscle layer of caput epididymis. In the female from TS24, the development of the oviduct, uterine horn and vagina are described. Oviduct and uterine horn are each divided into epithelial, muscle, outer layers and developing vasculature. The vagina is first divided into a lower and an upper part with each of these having the subcomponents epithelium, muscle layer and developing vasculature. The relative location along the length of the vagina may be unclear. Hence group terms have been created for epithelium of vagina, muscular layer of vagina and developing vasculature of vagina, as occurred with epididymis.
Annotation of the genital tubercle and resultant external genitalia
Annotation of the development of the genital tubercle to form the various parts of the external genitalia was based upon Haraguchi et al (2000), Haraguchi et al (2001), Perriton et al (2002) and Suzuki et al (2003). This process has also been reviewed in Yamada et al (2003) and Suzuki et al (2004). The external genitalia give rise to the urethral plate, which is then closed to form the caudal region of the urethra. However, these terms are included in the urinary system. The appearance of the genital tubercle is indistinguishable between males and females until around TS24, after which time the elements are the same but there are some recognisable differences in the preputial tissues. In both the male and female reproductive tracts from TS20, the genital tubercle is subdivided into the genital tubercle mesenchyme, genital tubercle surface epithelium and distal urethral epithelium (Haraguchi et al, 2001). From TS22, the genital tubercle, which will give rise to the penis and the clitoris, is subdivided into the distal genital tubercle and preputial swelling. Each of these consists of a surface epithelium and a mesenchyme layer. From this time, the labioscrotal swellings appear and are divided into mesenchyme and surface epithelium. From TS23, the terms male external genitalia and female external genitalia are used to describe the genital tubercle and the labioscrotal swellings. In the male at this time, the genital tubercle comprises the glans of the genital tubercle (syn: penis anlage) and the preputial swelling. The preputial gland is now annotated within the preputial swelling. In the female, the term female external genitalia includes the genital tubercle of female (syn:clitoris anlage) and the preputial swelling (syn: prepuce). Again, the preputial gland is present from this stage. At TS26, the male penis is subdivided into the glans penis, crus penis and prepuce. The scrotal swelling becomes the scrotal fold. In the TS26 female, the genital tubercle (syn: clitoris) is subdivided into the glans clitoris, crus of clitoris and prepuce. The labial swelling is now given the synonym labia.
1.10 Adult genitourinary ontology
The murine adult (TS28sm) urinary and reproductive ontology for male and female may be viewed at http://www.gudmap.org/Resources/Ontologies.html. The main distinctions between the adult and developmental portions of the ontology relate to the maturation of the reproductive system and the loss of nephrogenesis in the kidney. Of note in the male reproductive tract are significant elaborations of the testis, scrotum, prostate gland, bulbourethral gland and ejaculatory duct. In the testis the seminiferous cords become seminiferous tubules and all stages of maturation of the germ cells (spermatogonium, spermatocyte, spermatid, maturing spermatozoan) are present. The scrotum includes dermal and epidermal layers, including hair follicles and sweat glands. The prostate is subdivided into anterior, dorso-lateral and ventral prostate glands, each with glandular and ductal epithelia and interstitial components, including smooth muscle. Within the female reproductive tract, postnatal additions within the ovary include the various stages of folliculogenesis. The preantral (primordial, primary, secondary) follicles of the ovary are characterised by the growth and maturation of the oocyte. The antral (tertiary (syn: antral) and Graafian (syn: pre-ovulatory)) follicles of the ovary are characterised by their significant increase in size and the formation of an antrum or cavity. Each of these follicular stages is present in the ontology as are the atretic and ruptured follicle and the corpus luteum. Each stage of follicle includes the thecal cells, granulosa cells and the oocyte (aside from ruptured follicle). The oviduct is subdivided into infundibulum and rest of oviduct. The uterus includes the cervix uteri. Within the urinary system, nephrogenesis ceases within the first postnatal week in the mouse, and so the nephrogenic zone is absent and only mature nephrons are present. The outer medulla of the kidney is now divided into the outer stripe and inner stripe. As a consequence, the group term for loop of Henle now includes three components, the loop of Henle of outer stripe of outer medulla, the loop of Henle of inner stripe of outer medulla and the loop of Henle of inner medulla. The loop of Henle of outer stripe of outer medulla contains the proximal straight tubule (syn: pars recta) and the premacula segment of the distal straight tubule (syn: thick ascending limb), while the loop of Henle of inner stripe of outer medulla contains the thin descending limb and the premacular segment of the distral straight tubule (syn: thick ascending limb).
1.11 Potential for further development and application of the ontology
This ontology of the murine genitourinary tract will be readily useable by the renal, reproductive and urological community for the uniform descriptive annotation of expression patterns seen in the developing and adult genitourinary tract, whether assessed using RNA in situ hybridisation, immunohistochemistry, histochemistry, or microarray analysis (alone or on fine structure isolated by laser capture microdissection) and whether assessed in wildtype or mutant animals. The nature of the ontology is such that the level of detail included by the annotator can vary depending upon whether the user has access to low or high-resolution expression pattern data. In addition, the ontology is descriptive enough to be aligned with 2D and ultimately 3D models of the developing urinary tract across time to allow the development of queries based on structure / location and stage of development. The approach that has been taken here can readily be applied to other specific organ systems.
The ontology is a working version, which we anticipate will be modified both as use reveals existing deficiencies and analysis reveals novel gene expression patterns that point to new terms. There are terms in common use in the literature of kidney development for which a clear histological boundary is not present. The notable case in point is the Foxd1 / BF2-positive stroma of the kidney. At a histological level, the distinction between stroma and interstitium is not possible to discern, but one does not replace or encompass the other. While the ontology is currently largely based on anatomical descriptions, it is likely that gene expression patterns are yet to be discovered that will distinguish additional structural subcompartments not readily visible at a histological level. The nature of the ontology is such that it can be readily revised to accommodate new subcompartments, providing there are sufficient reference examples to assist in accurate annotation. There are many specific cell types known to exist in the subdivisions of the developing nephron. There are few cases in the current ontology in which individual cell types have been described. The exceptions include the germ cells, resident mononuclear cells, principal cells and intercalated cells. The collecting duct is known to contain three distinct cell types, including the intercalated, principal and medullary collecting duct cells. Specific transporters mark these cells and it has been shown that the loss of expression of genes can alter these cellular compartments. Blomqvist et al (2004) describe the loss of the intercalated cells of the collecting duct in mice lacking the Foxil transcription factor gene. The extension of this anatomical map into a molecular map will be gradually achieved for all distinct cell types once enough distinct gene expression patterns have been described. Individual cell types will form the terminal branches of existing terms in the ontology.
Having created an anatomical ontology for the genitourinary tract, it is now possible to layer onto this framework some additional ontological trees that need not coincide with the compartments recognised in the ontology. The challenge of creating multiple ‘part of’ ontologies layered over each other has been previously proposed and discussed in Burger et al (2004b). This might include a functional ontology including distinct islands of functional description without necessarily any lineage or location relationships. ‘Instance-of’’ links would enable us to represent functional relationships between elements of the genitourinary tract with tissue, cell type or functional descriptors in other parts of the embryo, such as secretory epithelium or stem cell. Lineage relationships revealed via lineage tracing studies, using promoter-Cre crossed to reporter mice, may also be mapped onto this framework.
The process of creating an ontology for the annotation of the murine genitourinary tract has raised a number of issues that may ultimately only be addressed once we have more examples of distinct patterns of gene expression. One of these is the maturation of the medullary region of the murine kidney. The medulla of a postnatal day 1 mouse still contains diffuse areas of medullary interstitial cells, suggesting that the counter-current mechanism for urinary concentration is not yet mature. A careful analysis of the P1 to P14 period in the mouse both by expression profiling and subsequent RNA in situ hybridisation is required to analyse this process more fully. It may then be necessary to alter the ontology such that there is additional information specific to some of these early postnatal periods. Hence, the ontology serves to generate and test hypotheses rather than existing solely as a descriptive tool.
In conclusion, we have generated an anatomical text-based ontology of the murine genitourinary tract that integrates into the existing Edinburgh Mouse Atlas Project ontology. The ultimate aim will be to create molecular lineage and functional ontologies that lie above this anatomical framework based upon the growing understanding of genitourinary development that is coming from in situ hybridisation, laser capture microdissection, expression profiling, lineage tracing and gene disruption using transgenesis.
2. Experimental procedures
2.1 Material collection and histology
Outbred or C57Bl6 mouse embryos were collected from TS17 (10.5dpc) to TS26 together with postnatal day 1 (P1) and adult. For histological analyses, tissue was fixed in 4% paraformaldehyde and processed and embedded in paraffin, sectioned at 4–7μm and stained with haematoxylin and eosin. For section in situ hybridisation, tissue was fixed in fresh 4% paraformaldehyde at 4°C for 16–24hrs, washed in PBS and stored in 70% ethanol at 4°C. For whole mount in situ hybridisation tissue was washed in PBTX and dehydrated with a PBTX/methanol series (25/75, 50/50, 75/25) and stored in 100% methanol at −20°C.
2.2 Whole mount in situ hybridisation
Whole mount in situ hybridisation was performed using digoxigenin (DIG)-labelled antisense riboprobes using the BioLane HTI Robot and is described in detail on the GUDMAP gene expression database (http://www.gudmap.org/Research/Protocols/Little.html). Briefly, tissue was rehydrated with a methanol/PBTX series, digested with Proteinase K (10μg/ml)/PBTX and re-fixed with 0.2% gluteraldehyde/4% paraformaldehyde. Tissue was washed, incubated in pre-hybridisation solution at 65°C for 2hrs and incubated overnight with riboprobe (0.2μg/ml) in pre-hybridisation solution at 65°C. Following post-hybridisation washes (Solution 1; SSC; 0.1% CHAPS; TBTX series), tissue was pre-blocked in 0.1%BSA/TBTX for 2hrs then incubated with pre-absorbed anti-DIG alkaline phosphatase Fab fragments (Roche). After the post-antibody washes (0.1% BSA/TBTX), samples were incubated with for 2–60hrs with chromogenic substrates NBT/BCIP or BM Purple to detect the hybridised alkaline phosphatase activity. Once the signal had reached optimal intensity (2–60hr), the tissues were washed (PBTX or PBS) and fixed in 4% paraformaldehyde/PBS at 25°C for 20min followed by PBS washes in order to preserve the signal.
The detailed protocol for whole mount in situ hybridisation of TS23 urogenital system (see Figure 4) can be found at http://www.gudmap.org/Research/Protocols/McMahon.html (Digoxigenin-Labeled In situ Hybridization for E15.5 Wholemount Kidneys). Briefly, TS23 urogenital system was dissected free of surrounding tissues and fixed with 4% paraformaldehyde at 4°C for 24 hours. Tissues were then washed with PBS, dehydrated with graded methanol and stored in 100% methanol at −20°C. Tissues were rehydrated, treated with proteinase K for 30 minutes, post-fixed and prehybridised before hybridisation with 500ng/ml riboprobes at 70°C overnight. Tissues were then washed, treated with RNase and incubated with anti-Digoxigenin antibody at 4°C overnight. After extensive washes, signals were detected with BM purple at room temperature. Colour reactions were examined at 3h, 6h, 9h, 12h, 24h, 36h and 48h and stopped when background starts to come up or signals are strong.
2.3 Paraffin section in situ hybridisation
Fixed tissue was paraffin-embedded and sectioned at 7μm. Paraffin section in situ hybridisation was performed using digoxigenin (DIG)-labelled antisense riboprobes using the Tecan Freedom Evo 150 Robot and is described in detail on the GUDMAP gene expression database (http://www.gudmap.org/Research/Protocols/Little.html). Briefly, following dewaxing and rehydration, sections were fixed with 4% paraformaldehyde, washed with PBS, assembled into slide chambers and inserted into the Tecan Freedom Evo 150 Robot. Sections were then treated at 25°C with 0.2N HCl for 5minutes followed by permeabilisation with Proteinase K (10μg/ml) for 20–30minutes, acetylated (0.5% acetic anhydride in 0.1M Tris. HCl pH8.0) and washed with 2xSSC. Sections were immersed in pre-hybridisation solution whilst the chamber racks were heated from 25°C to 64°C. Hybridisation occurred at 64°C for 10hours with 0.3–0.5μg/ml of riboprobe in hybridisation buffer (50% formamide, 2 x SSC, 10% dextran sulphate, 1x Denhardt’s, 0.2mg/ml yeast tRNA, 0.5mg/ml salmon sperm). After a series of SSC stringency washes, sections were blocked (20% sheep serum, 1xDIG blocking buffer) and incubated with 1:2000 of anti-DIG-alkaline phosphatase Fab fragments for 60minutes at 25°C. Sections were washed with Roche DIG Wash Buffer, NTMT (0.1M NaCl, 0.1M Tris. HCl pH9.6, 50mM·MgCl2, 0.1% Tween20) and NT (0.1M NaCl, 0.1M Tris. HCl pH9.6). Chromogenic substrates NBT/BCIP or BM Purple were used to detect the hybridised alkaline phosphatase activity. Once the signal had reached optimal intensity (4–60hr), the slides were rinsed and fixed in 4% paraformaldehyde/PBS at 25°C for 20min followed by PBS washes in order to preserve the signal.
2.4 Fresh frozen section in situ hybridisation
Neonatal kidneys were dissected free of all surrounding tissues except the ureter and fixed with 4%paraformaldehyde at 4°C for 24h. After PBS washes, they were incubated with 30%sucrose at 4°C overnight. Kidneys were swirled in 5 dishes of OCT to get rid of surface sucrose and mounted in OCT in dry ice/ethanol bath. The OCT blocks were stored at −80°C. Sections were cut at 20μm, post-fixed, treated with proteinase K and post-fixed again. Air dried sectioned were incubated with 500ng/ml riboprobes at 68°C overnight. Sections were then washed, treated with RNase and washed again with solutions of increasing stringency. After incubation with anti-Digoxigenin antibody at 4°C overnight, sections were washed and developed with BM purple for 7days. Colour reactions were stopped with fixation and sections were mounted with glycergel mounting medium. The detailed protocols for section in situ hybridization on neonatal kidneys can be found at http://www.gudmap.org/Research/Protocols/McMahon.html (Digoxigenin-Labeled In situ Hybridization for P1 Mouse Kidney Sections).
Supplementary Material
Supplementary Table 1. Anatomical ontology for the developing murine genitourinary tract from TS17 to TS19 presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 2. Anatomical ontology for the developing murine urinary tract from TS20 to TS27 presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 3. Anatomical ontology for the adult sexually mature (TS28sm) murine urinary tract presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 4. Anatomical ontology for the developing murine reproductive tract (male and female) presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 5. Anatomical ontology for the adult sexually mature (TS29sm) murine reproductive tract (male and female) presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Acknowledgments
We acknowledge Jonathan Bard for his role as curator of EMAP and Terry Hayamizu for advice on integration of this ontology into EMAP. We also thank Erik Christiansen, Jeff Christiansen, Lorna Richardson, Norah Spiers, Axel Thomson, Phillipa Saunders, Tom Spencer, Brigitte Kaissling, Wilhelm Kriz, Doris Herzlinger, Daine Alcorn, Larry Patterson, Christopher Tindal, Josephine Bowles and Dagmar Wilhelm for technical, intellectual and anatomical advice. We also thank other members of Genitourinary Development Murine Atlas Project (GUDMAP) for their advice and opinion. We acknowledge Grant Challen, Gemma Martinez, Kyra Woods, Linda Tongpao and Michael Lusis for example RNA in situ images and technical assistance. This work was supported by the National Institute of Digestion, Diabetes and Kidney Diseases, NIH, USA (DK070136-02 in Little laboratory, DK070181 in the McMahon laboratory, DK DK07020001 in Davidson / Davies laboratories) and the European Community as part of the European Renal Genome Project, EuReGene, (FP6 005085 in Davidson / Davies laboratories).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldock RA, et al. EMAP and EMAGE: a framework for understanding spatially organized data. Neuroinformatics. 2003;1(4):309–325. doi: 10.1385/NI:1:4:309. [DOI] [PubMed] [Google Scholar]
- Bard JBL, Kaufman MH, Dubreuil C, Brune RM, Burger A, Baldock RA, Davidson DR. An internet-accessible database of mouse developmental anatomy based on a systematic nomenclature. Mech Development. 1998;74:111–20. doi: 10.1016/s0925-4773(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Batourina E, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37(10):1082–9. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, et al. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest. 2004;113(11):1560–70. doi: 10.1172/JCI20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn. 2001;221(2):201–5. doi: 10.1002/dvdy.1134. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68(4):422–8. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Burger A, et al. Formalization of Mouse Embryo Anatomy. Bioinformatics. 2004a;20:259–267. doi: 10.1093/bioinformatics/btg400. [DOI] [PubMed] [Google Scholar]
- Burger A, et al. Integrating partonomic hierarchies in anatomy ontologies. BMC Bioinformatics. 2004b;5(1):184. doi: 10.1186/1471-2105-5-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B, et al. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev. 1999;84:127–131. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–52. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- Georgas K, et al. Characterisation of Crim1 expression in the developing mouse urogenital tract reveals a sexually dimorphic gonadal expression pattern. Dev Dyn. 2000;219(4):582–7. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1072>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gyftopoulos K, et al. Clinical and embryologic aspects of penile duplication and associated anomalies. Urology. 2002;60(4):675–9. doi: 10.1016/s0090-4295(02)01874-5. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, et al. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127(11):2471–9. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, et al. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128(21):4241–50. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Hoy WE, et al. Nephron number, hypertension, renal disease and renal failure. J Am Soc Nephrol. 2005;16:2557–2564. doi: 10.1681/ASN.2005020172. [DOI] [PubMed] [Google Scholar]
- Johkura K, et al. Immunohistochemical Localization of Peroxisomal Enzymes in Developing Rat Kidney Tissues. J Histochem Cytochem. 1998;46:1161–1174. doi: 10.1177/002215549804601008. [DOI] [PubMed] [Google Scholar]
- Kaobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev. 2003;4:969–80. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Kaufman M. The Atlas of Mouse Development. 2. Academic Press; London: 1994. [Google Scholar]
- Koopman P, et al. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–2. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. The ultrastructure of the developing proximal tubule in the rat kidney. J Ultrastruct Res. 1975;51:119–139. doi: 10.1016/s0022-5320(75)80013-x. [DOI] [PubMed] [Google Scholar]
- Larsson L, Maunsbach AB. The ultrastructural development of the glomerular filtration barrier in the rat kidney: a morphometric analysis. J Ultrastruct Res. 1980;2:392–406. doi: 10.1016/s0022-5320(80)90074-x. [DOI] [PubMed] [Google Scholar]
- Marlier A, Gilbert T. Expression of retinoic acid-synthesizing and –metabolizing enzymes during nephrogenesis in the rat. Gene Expr Patterns. 2004;5:179–85. doi: 10.1016/j.modgep.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Martineau J, et al. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–68. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Martinez G, Georgas K, Challen GA, Rumballe B, Davis MJ, Taylor D, Teasdale RD, Grimmond SM, Little MH. Definition and spatial annotation of the dynamic secretome during early kidney development. Dev Dyn. 2006;235:1709–19. doi: 10.1002/dvdy.20740. [DOI] [PubMed] [Google Scholar]
- Menke DB, et al. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–12. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Mitchell EK, et al. Differential gene expression in the developing mouse ureter. Gene Expr Patterns. 2006;6:519–38. doi: 10.1016/j.modgep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Nakai S, et al. Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development. 2003;130:4751–9. doi: 10.1242/dev.00666. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, et al. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990;109:911–23. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Perriton C, et al. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Potter EL. Normal and abnormal development of the kidney 1972 [Google Scholar]
- Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits. Sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978;39:90–100. [PubMed] [Google Scholar]
- Saino K, et al. Differential regulation of two sets of mesonephric tubules by WT1. Development. 1997;124:1293–99. doi: 10.1242/dev.124.7.1293. [DOI] [PubMed] [Google Scholar]
- Scholer HR, et al. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989;8:2543–50. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, et al. Regulation of outgrowth and apoptosis for the terminal appendage, external genitalia, development by concerted functions of Bmp signalling. Development. 2003;131:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- Suzuki K, et al. Development of the mouse external genitalia: unique model of organogenesis. Adv Exp Med Biol. 2004;545:159–72. doi: 10.1007/978-1-4419-8995-6_10. [DOI] [PubMed] [Google Scholar]
- Theiler K. The House Mouse: Atlas of Embryonic Development. New York: Springer-Verlag, New York; 1989. [Google Scholar]
- Warne SA, et al. Long-term urological outcome of patients presenting with persistent cloaca. J Urol. 2002;168(4 Pt 2):1859–62. doi: 10.1097/01.ju.0000030712.17096.0d. [DOI] [PubMed] [Google Scholar]
- Weizer AZ, et al. Determining the incidence of horseshoe kidney from radiographic data at a single institution. J Urol. 2002;170(5):1722–6. doi: 10.1097/01.ju.0000092537.96414.4a. [DOI] [PubMed] [Google Scholar]
- Yamada G, et al. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–60. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yamada G. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn. 2006;235:1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]
- Yin Y, Ma L. Development of the mammalian female reproductive tract. J Biochem. 2006;137:677–83. doi: 10.1093/jb/mvi087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Anatomical ontology for the developing murine genitourinary tract from TS17 to TS19 presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 2. Anatomical ontology for the developing murine urinary tract from TS20 to TS27 presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 3. Anatomical ontology for the adult sexually mature (TS28sm) murine urinary tract presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 4. Anatomical ontology for the developing murine reproductive tract (male and female) presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term
Supplementary Table 5. Anatomical ontology for the adult sexually mature (TS29sm) murine reproductive tract (male and female) presented as a flat file view and indicating unique EMAP identification numbers for each term within the ontology. syn: synonym, GROUP: group term