Abstract
The use of exhaled nitric oxide measurements (FEno) in clinical practice is now coming of age. There are a number of theoretical and practical factors which have brought this about. Firstly, FEno is a good surrogate marker for eosinophilic airway inflammation. High FEno levels may be used to distinguish eosinophilic from non‐eosinophilic pathologies. This information complements conventional pulmonary function testing in the assessment of patients with non‐specific respiratory symptoms. Secondly, eosinophilic airway inflammation is steroid responsive. There are now sufficient data to justify the claim that FEno measurements may be used successfully to identify and monitor steroid response as well as steroid requirements in the diagnosis and management of airways disease. FEno measurements are also helpful in identifying patients who do/do not require ongoing treatment with inhaled steroids. Thirdly, portable nitric oxide analysers are now available, making routine testing a practical possibility. However, a number of issues still need to be resolved, including the diagnostic role of FEno in preschool children and the use of reference values versus individual FEno profiles in managing patients with difficult or severe asthma.
Keywords: exhaled nitric oxide, asthma, diagnosis, monitoring, treatment
It is now 12 years since it was first reported that exhaled nitric oxide (NO) levels are increased in bronchial asthma.1 This discovery followed a period of intense interest in the biology of NO during the late 1980s.2 The numerous roles of NO in respiratory pathophysiology have been extensively reviewed.3 NO is an endogenous messenger with a diverse range of effects including non‐adrenergic, non‐cholinergic neurotransmission, vascular and non‐vascular smooth muscle relaxation.3
There is contradictory evidence regarding the exact function of NO in lung disease. In pathological situations NO is a pro‐inflammatory mediator with immunomodulatory effects.3 This appears to predispose to the development of airway hyperresponsiveness (AHR), although this is not a consistent finding.3,4 On the other hand, under physiological conditions NO acts as a weak mediator of smooth muscle relaxation and protects against AHR.5 In exhaled air NO appears to originate in the airway epithelium.6 Although raised levels may occur with a number of airway or lung diseases,7 the most important context in which the measurement of NO is clinically useful is that of allergic airways disease.
It is against this background that measuring the fraction of NO in exhaled air (Feno) has emerged as a potentially important clinical tool. Feno can be measured easily using a range of commercially available analysers, and smaller less costly devices are now becoming available. This opens the possibility that Feno measurements might be used routinely in the assessment of airway disease. This is a significant advance. To date, assessing airway physiology—that is, changes in airway calibre and/or bronchodilator or bronchoconstrictor responsiveness—has been the principal means of providing supportive evidence for the diagnosis of airways disease and assessing severity. Although pulmonary function tests will always remain important, they are one step removed from the issue of interest—that is, airway inflammation. Thus, Feno measurements provide a complementary and, in some instances, a more relevant perspective.
In this paper we will address key issues of importance to both adult and paediatric respiratory clinicians who are contemplating using Feno measurements in day to day practice.
Rationale for the use and interpretation of Feno measurements
A number of lines of evidence converge to provide the rationale for using Feno measurements in the assessment and management of respiratory disease. There are two key points: (1) there is a highly significant relationship between Feno and eosinophilic airway inflammation, and (2) there is an equally important relationship between eosinophilic airway inflammation and steroid responsiveness. The evidence is summarised as follows:
Feno measurements are highly correlated with eosinophilic airway inflammation.
Eosinophilic airway inflammation is associated with a positive response to steroid treatment.
Raised Feno levels predict steroid responsiveness in patients with non‐specific respiratory symptoms.
The use of inhaled corticosteroid (ICS) treatment in asthma results in a fall in Feno, and there is a dose‐dependent relationship between ICS and Feno.
Feno measurements are highly correlated with eosinophilic airway inflammation
Atopic asthma is characterised by an inflammatory infiltrate in the airways, with a predominance of mast cells and eosinophils.8 Studies confirm that Feno measurements correlate well with airway eosinophilia in induced sputum,9,10 biopsy material,11,12,13 and bronchoalveolar lavage fluid.14 In one study a significant relationship between Feno and blood eosinophils was also reported.15 A similar relationship has been described between Feno and sputum eosinophilic cationic protein16 in patients with asthma.
Importantly, two studies have shown that the relationship between Feno levels and airway eosinophilia is independent of the clinical diagnosis. It has been reported in patients with chronic obstructive pulmonary disease (COPD).17 In the study by Brightling et al11 patients who did not fulfil criteria for the diagnosis of asthma but who had eosinophilic bronchitis had raised Feno levels. In atopic patients with allergic rhinitis but no asthma, Feno levels are also raised.18,19,20 Similarly, in atopic asthmatic subjects in remission for many years, but who nevertheless have eosinophilic airway inflammation in bronchial biopsies, Feno levels are increased.13 All of these data form the basis on which Feno measurements are considered reliable as a non‐invasive marker of eosinophilic airway inflammation.
Eosinophilic airway inflammation is associated with a positive response to steroid treatment
Treatment with corticosteroids results in a reduction in airway eosinophilia in asthma and a simultaneous improvement in clinical parameters.21,22 In contrast, in asthma which is not characterised by eosinophilia (at least in sputum), the response to steroids is likely to be poor.23,24 These findings also apply in patients with fixed airflow obstruction in whom neither the history nor physiological measurements permit easy discrimination between asthma and COPD. In such patients, a positive outcome with a trial of steroid treatment is associated with the presence of sputum eosinophilia.25,26 Thus, assessing the character of airway inflammation (eosinophilia) appears to be important in the initial management of patients with chronic respiratory symptoms in order to identify those who are more likely to benefit from treatment with steroids.
Raised Feno levels predict steroid responsiveness in patients with non‐specific respiratory symptoms
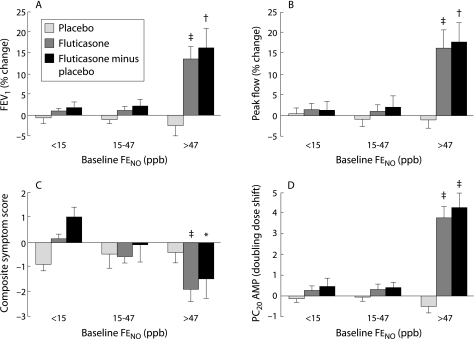
Little et al27 have shown that the clinical benefit of increased steroid treatment in patients with asthma is greatest in patients with raised Feno levels. This has been taken a step further by Smith et al28 who evaluated the predictive accuracy of Feno measurements (as a surrogate for airway eosinophilia) in adult patients with undiagnosed respiratory symptoms. In that study, the positive and negative predictive values for a range of outcomes following a trial of inhaled fluticasone were superior for Feno as a predictor than spirometry, bronchodilator response, and measurements of AHR. Importantly, this study identified an optimum cut point for steroid response at an Feno of 47 ppb (fig 1). This outcome was largely independent of the final diagnosis. A similar result has been reported by Szefler et al29 who showed that children with high Feno values are more likely to respond to ICS than children with lower Feno values.
Figure 1 Steroid responsiveness in relation to Feno measurements in patients with non‐specific chronic respiratory symptoms. Mean (SE) changes from baseline in (A) forced expiratory volume in 1 second (FEV1), (B) morning peak flow over last 7 days of treatment, (C) composite symptom score, and (D) provocative concentration of adenosine monophosphate causing a 20% fall in FEV1 (PC20AMP) following treatment with inhaled fluticasone 500 µg/day (minus change with placebo), stratified by baseline Feno expressed as tertiles. Comparisons between tertiles were performed using one way analysis of variance with linear contrasts to identify any trend across the three tertiles; *p<0.05; †p<0.01; ‡p<0.001. Reproduced from Smith et al28 with permission of the publishers.
ICS treatment in asthma results in a fall in Feno with a dose‐dependent relationship between ICS and Feno
A number of studies have shown that ICS treatment results in a fall in Feno levels in patients with mild asthma.30,31,32 Both the magnitude and the time interval over which the reduction occurs are dose‐dependent33,34 and the response is reproducible.35 Feno levels tend to plateau at higher doses of ICS.36 In addition, there are highly significant correlations between the changes in Feno and changes in induced sputum eosinophils with ICS therapy.37
Taken together, these data provide foundational evidence that Feno measurements have a potentially important role in evaluating and treating patients with airways disease. Firstly, Feno may be used as a surrogate marker for airway diseases characterised by eosinophilia such as atopic asthma, cough variant asthma, and eosinophilic bronchitis. Secondly, because of the close relationship between steroid responsiveness and airway eosinophilia (in contrast to other histological phenotypes), Feno measurements have a role in predicting and monitoring the response to ICS treatment.
Diagnosing airways disease
Establishing a diagnosis is the first step in clinical management but, for diseases of the airways, a diagnostic label has its limitations. The term “chronic obstructive airways disease” (COPD) encompasses a spectrum of overlapping pathologies and the phenotype is a mixed one, especially in relation to treatment. The same is true for bronchial asthma, which is increasingly acknowledged to be heterogeneous,38,39 particularly if it is severe.40,41 Against this background, and given the specificity of Feno measurements as a marker for eosinophilic airway inflammation, it is not surprising that Feno offers advantages as well as limitations as a “test for asthma”.
Asthma
In adults, Feno measurements are helpful in discriminating asthma from non‐asthma.42 It is best to reserve the test for investigating chronic symptoms (of 6 weeks duration or longer) because viral illness may give rise to a false positive result.43,44 In the study by Dupont et al,45 among 240 non‐smoking steroid naïve individuals of whom 160 (67%) fulfilled the criteria for the diagnosis of asthma, Feno levels were highly predictive of asthma with a sensitivity and specificity of 85% and 90%, respectively. In the study by Smith et al,46 similar sensitivity (88%) and specificity (79%) were obtained in 47 patients of whom 17 had asthma. Predictive values were almost identical to those obtained using induced sputum cell counts. A striking feature in that study was the poor performance of almost all the “conventional” diagnostic tests against which Feno measurements were compared. This reflects the fact that, in unselected patients, most will have mild disease with normal lung function. In this setting Feno measurements may therefore be more relevant than traditional lung function tests. Interestingly, the combination of a raised Feno (>33 ppb) and abnormal spirometry (FEV1 <80% predicted) provides even greater sensitivity (94%) and specificity (93%) for the diagnosis of asthma.46,47
It is important to remember that patients may fulfil conventional clinical criteria for the diagnosis of asthma and yet Feno levels will be normal, especially in non‐atopic subjects. Normal values do not exclude the diagnosis of asthma. Measuring AHR may reveal a positive clinically relevant result. Thus, Feno measurements complement AHR rather being a substitute for it, both in population surveys48 and in patients with asthma.49 This highlights further the fact that the asthma phenotype is heterogeneous, and that Feno measurements provide a perspective on only one aspect of the “asthma syndrome”.
Non‐specific respiratory symptoms
Feno measurements have a wider role in assessing patients with undiagnosed chronic respiratory symptoms. There is a broad differential diagnosis in such patients depending on age. It includes eosinophilic bronchitis, cough variant asthma, post‐viral bronchial hyperresponsiveness, postnasal drip and other ENT problems, gastro‐oesophageal reflux disease, vocal cord dysfunction, primary hyperventilation syndrome, and COPD. In children, recurrent wheezy bronchitis, cystic fibrosis, congenital abnormalities of the airways or lungs, and primary ciliary dyskinesia also need to be considered.
FEno measurements may also permit the clinician to anticipate treatment responses. For eosinophilic bronchitis and cough variant asthma, which are characterised by eosinophilic airway inflammation and increased Feno levels, a positive response to a trial of steroid treatment is likely.11,50 On the other hand, for other diagnoses—for example, vocal cord dysfunction presenting as “asthma” which clinicians often treat empirically with steroids with little meaningful benefit51—it is just as helpful to have a low normal Feno level indicating a condition which is not characterised by eosinophilic airway inflammation and, in turn, is less likely to respond to steroids.
Preschool children
Given that spirometry and sputum induction cannot easily be performed in preschool children, a non‐invasive measurement of airway inflammation is potentially very useful. As the single breath technique for measuring Feno in this age group is not suitable in preschool children, several alternatives have been developed, varying from modifications of the standard online technique to offline tidal breathing methods without flow control.52,53,54,55,56,57,58,59,60,61 In general, these techniques (which were reviewed by an ERS/ATS Task Force) are less sensitive in discriminating between asthmatic and non‐asthmatic subjects.62,63
Evidence as to the overall diagnostic usefulness of Feno measurements in young children is mixed. In an unselected population of preschool children too young to perform spirometric tests, Feno performed poorly in distinguishing between asthma and non‐asthma. Differences in Feno values between atopic children, children with doctor diagnosed asthma, and healthy children were less pronounced than in older subjects.64 However, when used in selected children, the performance characteristics are somewhat improved.65
In the differential diagnosis of non‐specific respiratory symptoms, the same issues are encountered. Baraldi et al studied a group of 13 young children with recurrent wheeze and compared their Feno values with those of nine healthy controls and six children with a first episode of wheezing.53 Exhaled air was collected offline in a bag during tidal breathing without flow control. During an acute episode, Feno was significantly higher in those with recurrent wheeze than in controls, while in children with their first episode of wheezing Feno levels did not differ from normal children. These data are in keeping with those of Ratjen et al60 who measured peak Feno values online in mixed exhaled air (from mouth and nose).
A test that might allow better targeting of anti‐inflammatory treatment, particularly in preschool children, would be very helpful. Feno is a promising tool in this regard. Treating infants and young children with recurrent wheeze and increased Feno levels with corticosteroids reduced Feno to normal or near normal values.53,66 Also, montelukast reduced Feno values in young children with early onset asthma.67,68
Influence of atopy
Several epidemiological studies have confirmed that Feno levels are raised in atopic subjects, whether or not they have significant lower respiratory tract symptoms.18,20,64,69,70,71,72 There is also a strong correlation between Feno levels and total as well as antigen specific IgE.71,72 This overall picture may be explained by the fact that even asymptomatic atopic patients may have mild airway inflammation,13,73 giving rise to increased Feno levels.
It has been suggested that the usefulness of Feno measurements may be limited only to atopic subjects, but we disagree with this view. Firstly, not all atopic individuals are identified using skin prick testing—that is, the label “non‐atopic” may be falsely negative. Secondly, the presence of a low/normal Feno level in patients with chronic respiratory symptoms may be equally helpful in pointing the clinician away from the diagnosis of an atopic condition. In practice, when raised Feno levels are encountered in atopic subjects, additional investigations or treatment should be based on a history of significant symptoms. There is little evidence at present to support intervention in asymptomatic individuals.
Chronic obstructive pulmonary disease (COPD)
FEno levels are inconsistent in patients with COPD. This may be due to the confounding effect of current smoking or it may reflect the heterogeneity of underlying airway inflammation. Some studies report no significant change in Feno levels compared with controls,74,75 while others report that levels are increased.76 More recent evidence suggests that measuring alveolar rather than airway Feno may yield more important information,77 but at present this is technically demanding and beyond the scope of routine laboratory testing.
In older patients (>45 years) with fixed airflow obstruction, physiological tests alone are unhelpful in distinguishing those with asthma who would otherwise be classified as having COPD. Fabbri et al17 have shown that patients with historical evidence of asthma have eosinophilic airway inflammation in association with raised Feno levels. Earlier, Papi et al78 reported that increased sputum eosinophils and Feno levels occur in COPD patients with greater degrees of bronchodilator reversibility.
Perhaps the most important question is not whether the diagnostic label is accurate, but whether the response to anti‐inflammatory treatment can be predicted. The data provided by Brightling et al,26 in which induced sputum eosinophil counts were used as the predictor, are encouraging. In that study 22 of 67 patients with COPD whose induced sputum eosinophil count was in the uppermost tertile (>4.5%) had significant symptomatic as well as physiological improvements with oral prednisone. Using Feno measurements, Zietkowski et al76 showed that the increase in post‐bronchodilator FEV1 after 2 months of open label treatment with inhaled budesonide 800 μg/day was strongly correlated (r = 0.73, p = 0.0003) with baseline Feno levels in 19 ex‐smoking patients with COPD. However, statistically significant correlations are not the same as predictive accuracy. There is a need for more work to be done to establish the exact role of Feno measurements in assessing COPD. This will require larger randomised controlled studies.
Other diseases in which Feno may have a role
Besides the common airways diseases, Feno measurements may have a role in the assessment of several other respiratory and non‐respiratory conditions (table 1).
Table 1 Respiratory and non‐respiratory conditions in which Feno measurements may have a role in diagnosis.
| Increased Feno | Variable changes in Feno reported | Decreased Feno |
|---|---|---|
| Asthma1,79Late asthmatic response80,81Allergic rhinitis19Viral infections43,44,82Hepatopulmonary syndrome83Liver cirrhosis84,85Acute/chronic rejection of lung transplant including bronchiolitis obliterans86,87,88,89,90 | Bronchiectasis91,92,93COPD17,75,78,94,95,96,97,98,99,100,101,102Fibrosing alveolitis103Sarcoidosis104Systemic sclerosis105,106,107 | Cystic fibrosis91,108,109,110Primary ciliary dyskinesia111,112Pulmonary hypertension113HIV infection114ARDS115 |
Cystic fibrosis (CF)
In patients with CF, Feno measurements have not been found to be clinically helpful. Values are usually normal or low.108,109,110 There are several possible explanations. Firstly, there is decreased expression of nitric oxide synthase (NOS‐2) in patients with CF.116,117 Secondly, increased levels of nitrite are found in the breath condensate of patients with CF, suggesting trapping and metabolism of NO in secretions and mucus in CF airways.118,119
Primary ciliary dyskinesia (PCD)
Feno levels are significantly lower in patients with PCD than in healthy individuals, although with some overlap.111,120,121 Moreover, nasal NO (nNO) is extremely low in patients with PCD of all ages, and discriminates fully between affected and unaffected individuals. Measurement of nNO is likely to become the screening tool of choice.122 The diagnostic sensitivities and specificities of nNO for PCD range from 89% to 100% and from 97% to 100%, respectively. Low Feno and nNO levels may also be found in subjects with non‐PCD bronchiectasis and sinus disease.112,121
Again, there are several possible explanations. Firstly, there may be decreased NOS activity. Administration of l‐arginine as a substrate for NO increases nasal and exhaled NO formation in PCD, although not to normal values.123,124 This favours decreased NOS activity as a mechanism. Secondly, mucus may impair the diffusion of NO from the sinuses to the nasal cavity or from epithelial cells to the airway lumen, or may alter NO elimination.125 However, even in young infants with PCD, nNO is low, favouring the first explanation.126
NO is probably involved in stimulating ciliary motility.127 Nasal NO may also play a role in non‐specific host defences, including direct toxic effects on micro‐organisms.128 Reduced endogenous NO production and damage to NO producing cells may therefore contribute to recurrent airway infections.
Lung transplantation
Feno levels are increased in post‐transplant patients with unstable lung function.87,88 More recent studies have investigated whether sequential Feno measurements can identify patients with progressive bronchiolitis obliterans syndrome (BOS).89,90 In a study by Brugiere et al,90 mean Feno levels were twice as high in patients with progressive BOS than in those with or without BOS whose lung function remained stable over 14 months. Verleden et al89 evaluated the performance characteristics of Feno measurements over 2 years and obtained sensitivity, specificity, positive and negative predictive values for BOS of 92%, 84%, 80%, and 94%, respectively. The cut point used was 15 ppb (at an expiratory flow rate of 200 ml/s). This was equivalent to the upper limit of the 95% confidence interval for mean Feno levels in stable transplant patients. Interestingly, increased Feno levels preceded the changes in lung function by approximately 9 months. Although promising, the exact role of Feno measurements in post‐transplant monitoring is not yet established.
Feno measurements in the management of chronic asthma
Two important questions have emerged regarding Feno measurements in the ongoing management of asthma:
Does Feno have prognostic significance?
Can Feno be used to guide treatment decisions relating to anti‐inflammatory treatment?
Predicting exacerbations
Asthma is characterised by relapses and remissions, with deterioration in control provoked by a number of triggers as well as due to poor compliance with anti‐inflammatory therapy. There is a perceived need for an objective measurement which might provide warning of impending deterioration or the need to change treatment. Peak flow measurements have been used to fulfil this role, but with limited success because changes in peak flow largely coincide with deteriorating symptoms rather than precede them.
Overall, the prognostic value of Feno measurements to predict deteriorating asthma appears limited. In a small study involving a steroid reduction protocol, Jatakanon et al129 reported that changes in sputum eosinophils were superior to Feno measurements in predicting loss of control. In a study by Jones et al37 measurements of AHR to hypertonic saline, sputum eosinophils, and Feno measurements all ranked similarly as predictors of control in 78 asthma patients following ICS withdrawal. Sensitivities ranged from 21% (eosinophils >4%) to 65% (Feno >10 ppb at a flow rate of 250 ml/s), although positive predictive values were in the range of 80–88%. Interestingly, the measurement of changes in these measurements was only marginally better than using single measurements. An increase in Feno of 60% was deemed to be optimum, but this was only 50% sensitive with a positive predictive value of 83%. These studies used a steroid withdrawal protocol to mimic a clinical exacerbation and are not necessarily ideal. In a much smaller study Harkin et al130 reported that, in routine practice, increased levels of Feno predicted an exacerbation within the following 2 weeks. It may be that, with the advent of portable monitoring, daily Feno measurements may prove to be beneficial in anticipating deteriorating asthma. However, as yet no data are available.
Predicting the outcome of ICS withdrawal in stable asthma
A relevant question is whether markers of airway inflammation can be used to predict the successful reduction or withdrawal of ICS treatment. In studies by Leuppi et al131 and Deykin et al,132 while sputum eosinophil counts (>0.8%) were highly predictive of subsequent loss of asthma control over periods of 6 months and 16 weeks respectively, no prognostic significance could be derived from Feno measurements. In the first study,131 baseline rather than sequential Feno values were used in the calculations. In the second, the number of patients in whom Feno values were obtained was limited, making valid comparisons difficult.132 In the study by Zacharasiewicz et al133 the negative predictive value of sputum eosinophils (at a cut point of 0%) was 100%—that is, treatment reduction/withdrawal was 100% successful (during the subsequent 8 weeks) when sputum eosinophilia was absent. A negative predictive value of 92% was obtained for Feno at a cut point of 22 ppb or less. Focusing on Feno, Pijnenburg et al134 reported that, following steroid withdrawal in currently asymptomatic children, Feno levels 2 and 4 weeks later were highly predictive of relapse during the subsequent 24 weeks of follow up, with a cut point for Feno of 49 ppb providing best predictive accuracy—that is, Feno levels above this threshold predicted likely asthma relapse.
Taken together, we can conclude that sputum eosinophil counts (>1%) probably offer superior prognostic accuracy when evaluating whether or not patients require ongoing ICS treatment. Furthermore, in circumstances where induced sputum cannot be obtained (in the majority of centres and in young children), a high Feno level (>50 ppb) is likely to predict asthma relapse and a low Feno level (<20 ppb in children, <25 ppb in adults) is likely to predict asthma stability if measured at least 4 weeks after ICS treatment is reduced/withdrawn in a currently asymptomatic patient. The outcome in those with an intermediate result (Feno 20–50 ppb) is less certain.
Adjustment of ICS dose
Several studies have recently explored whether “inflammometry” can be used to optimise the dose of ICS treatment. Using induced sputum eosinophil counts, Green et al24 showed that a management strategy which prescribed a stepwise reduction in ICS dose if sputum eosinophils were <1% (or an increase in dose if >3%) reduced asthma exacerbations to 32% of the rate observed in the control group. In another randomised study Jayaram et al133 have shown that, when ICS treatment is adjusted to maintain sputum eosinophils below 2%, the risk of eosinophilic exacerbations was reduced significantly (by 49%), with the number requiring intervention with prednisone reduced by two thirds.135 Interestingly, in that study the benefits of the “inflammometry” strategy occurred predominantly in patients with moderate or severe asthma.
The underlying rationale for each of these studies is both plausible and desirable—that is, anti‐inflammatory treatment should be adjusted to ensure minimum airway inflammation. Currently, clinicians respond to uncontrolled symptoms or impaired lung function assuming that this results from uncontrolled airway inflammation. But the correlation between airway inflammation and either symptoms13,136 or lung function is weak,15,137 so the use of these end points to guide treatment can only be regarded as second best. It is far more rational that the ICS dose should be adjusted to take account of the principal action of ICS treatment—namely, control of airway inflammation.
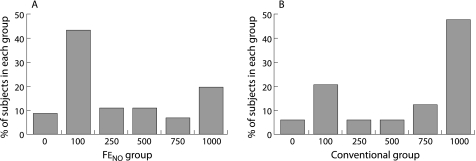
Two randomised controlled trials in which Feno measurements have been used to guide long term treatment with ICS have recently been published.32,138 In both studies significant but differing benefits were obtained using an Feno based algorithm. In a study of 94 adult asthmatic patients by Smith et al,138 a 40% reduction in ICS dose requirements was achieved using Feno measurements at a single cut point of 35 ppb without any significant difference in the rate of asthma exacerbations (fig 2). In a study by Pijnenburg et al32 comprising 85 children with atopic asthma, the cut point for Feno levels was similar at 30 ppb; there was no difference in cumulative ICS use between the Feno and control groups. However, in the Feno group there was a significant reduction in the severity of AHR, with a concomitant (but, for reasons of study size, non‐significant) reduction in exacerbations requiring oral prednisone.
Figure 2 Profile of dose distribution for mean inhaled fluticasone requirements over 12 months in 48 patients (conventional group) whose ICS dose was adjusted using a priori guidelines and 46 patients in whom the ICS dose was adjusted on the basis of Feno measurements (FeNO group; cut point equivalent to 35 ppb). There was a highly significant difference between the two groups (p = 0.008).138
Variation in the dose titration protocols and the study end points may account for the apparent differences in the results. However, overall, these studies provide encouraging evidence regarding the use of Feno in this clinical setting, although for the time being they must be regarded as indicative rather than conclusive. Firstly, in the study by Smith et al138 a single cut point for Feno was used to prompt either an increase or a decrease in ICS dose. However, a “one size fits all” approach may not be appropriate in regular clinical practice. Furthermore, although single cut points are appropriate in “back titration” studies with a high starting dose of ICS, two cut points defining three management choices (increase, no change or decrease in dose) may be more effective. This concept was included in the algorithm used by Pijnenburg et al32 but, clearly, this is an area which requires further investigation. Secondly, although the cut point for Feno was similar in both studies, substantially different criteria were used to guide ICS dose adjustment in the control groups. Irrespective of where the cut points are set, these will significantly determine the outcome in any dose adjustment strategy. This may explain why differing yet beneficial outcomes have been achieved in the “strategy” groups in the studies reported to date.24,32,138
An important and as yet unresolved issue is whether Feno measurements should be used for both upwards as well as downwards ICS dose titration. Clearly, the withdrawal of unnecessary ICS treatment or reducing excessive doses is an important goal of Feno monitoring. This was the primary objective in the study by Pijnenburg et al.32 In the study by Smith et al138 ICS dose reduction was a secondary end point but proved to be the most important outcome. Thus, data to date suggest that dose reduction is a fairly achievable objective. However, in patients with persistently high Feno levels (>50 ppb), it remains to be determined whether increasing the dose of ICS further will prove to be successful. It is doubtful whether it is justified if the patient is asymptomatic. However, although abolishing symptoms is a valid objective, it is not the only one. Higher doses of ICS reduce the frequency and impact of asthma exacerbations, and it may be that persistently raised Feno levels represent the signal to prescribe higher ICS doses with this objective in mind. This is controversial. On the one hand, the effect of ICS dose increments in the higher range on Feno is often small. Also, it is often not possible to normalise Feno values in patients with persistently high Feno levels, even on maximum doses.139 Equally, the cost‐benefit ratio for very high doses of ICS in asthma increases. Thus, until further studies have been completed, it seems prudent to put the emphasis on dose reduction when Feno levels are low (<25 ppb). Only if asthma is poorly controlled and issues of poor compliance and/or poor inhaler technique have been addressed should high Feno levels prompt an increase in ICS dose.
Measuring and interpreting Feno
Obtaining Feno measurements using commercially available analysers is, like riding a bicycle, fairly straightforward when you know how. The majority are “online”—that is, the analysis of exhaled gas is immediate and a result is available within a few minutes. Updated recommendations have recently been published and the reader is strongly advised to refer to them for fuller information.62,63 Establishing reference values for Feno measurements has been an evolving issue and remains problematic. Most of the studies conducted up to the year 2000 did not include a standardised method for Feno measurement. This severely limits their generalisablity. Further, our knowledge of factors which affect Feno levels in health and disease has expanded steadily. Demographic factors include age (adult versus paediatric) and smoking status.140,141,142 There is still some disagreement about whether atopy143 and sex144,145,146 are consistently important.
Normal values and clinically important changes
Against this background, current guidelines do not yet specify “normal” values.63 However, several recent studies have attempted to provide reference ranges for adults142,144,145 and children.145 In the study by Olin et al146 the interquartile range for Feno in healthy adults was 11.9–22.4 ppb. In a study comprising 30 healthy non‐atopic adult subjects, the upper limit of normal (mean plus two standard deviations) was 33.1 ppb.146 In the study by Buchvald et al145 in a population of 405 children, the upper limit of the 95% confidence interval was age dependent, ranging from 15.7 ppb at the age of 4 years to 25.2 ppb for adolescents. The reason for this age dependency is unknown, but may be related to increasing airway surface area with age, age dependent induction of NOS secondary to recurrent immunological stimulation, or the progressive reduction over time of a constant exhalation flow rate which is relatively high in younger children. For the time being at least, it is reasonable that upper limits of normal for healthy adults and school age children should be set at 33 ppb and 25 ppb, respectively.
The reproducibility of Feno measurements is excellent with a very high intraclass correlation (>0.9) for repeated within sitting measurements.146 This translates into a within subject standard deviation for repeated measurements of 2.1 ppb for adults146 and 1.6 ppb for children145 (both at a flow rate of 50 ml/s). Similar high degrees of reproducibility have been reported by other authors.86,147,148,149
In clinical practice, as far as repeated measurements are concerned, the population of greatest interest are patients with chronic asthma. In this group the coefficient of variation for within subject between sitting measurements ranges from 10.5%37 to 26%.148 There is no “normal range” for patients with asthma. However, anecdotally, it would appear that even when asthma is well controlled, non‐asthmatic “normal” Feno levels are rarely achieved. Thus, it may be that Feno levels measured when asthma is stable become the baseline reference point for individual patients against which subsequent measurements are weighed.
Kharitonov et al146 have reported that an absolute change of >4 ppb is significant. Based on the study by Ekroos et al,148 a percentage change of >26% would be deemed statistically significant. But are absolute or percentage changes of >4 ppb or >26% clinically important? And, if not, what constitutes a clinically meaningful change? In the study by Jones et al37 the median change in Feno which occurred between stability and “loss of control” after withdrawal of ICS treatment was 16.9 ppb (mean 24.9), but with a very large range (−10 to +141 ppb). The predictive accuracy of a change from baseline of 60% or greater was limited. These data suggest that group mean data may not be helpful in determining a clinically relevant change in individual patients. Further work is required to address this issue, particularly as to the changes (absolute and percentage) which might be anticipated in patients experiencing an asthma exacerbation while continuing to take regular controller therapy.
Interpretation
Based on currently available data, we have recently developed two algorithms for interpreting Feno results in day to day practice—one for diagnostic use and the other for ongoing asthma management. These are shown in tables 2 and 3.
Table 2 Feno levels as an aid to diagnosis of chronic respiratory symptoms.
| Feno (ppb) | Range | Eosinophilic airway inflammation | Interpretation (as an aid to diagnosis of chronic respiratory symptoms) | |
|---|---|---|---|---|
| Adults | Children | |||
| 5 | Low (<20 ppb if 12 years or younger; <25 ppb for adults) | Unlikely | Consider:Neutrophilic asthmaAnxiety/hyperventilationVocal cord dysfunctionRhinosinusitisGastro‐oesophageal refluxCardiac disease | Consider:Wheezy bronchitisGastro‐oesophageal refluxENT disordersCystic fibrosisPrimary ciliary dyskinesia (FEno <5 ppb), (check nasal NO)Congenital abnormalities, e.g. airway malaciaOther immunodeficiencies |
| 10 | ||||
| 15 | ||||
| 20 | ||||
| 25 | ||||
| 30354045 | Intermediate | Present but mild | Interpretation based on clinical presentation | Interpretation based on clinical presentation |
| 50556065Higher levels | High | Significant | Consider:Atopic asthma if the history is appropriate. If FEV1 <80% predicted, diagnosis of asthma is very likelyEosinophilic bronchitisChurg‐Strauss syndromeA positive response to a trial of inhaled or oral steroid is likely. In ex‐smokers with COPD this may also be true | If combined with any objective evidence of reversible airway obstruction, asthma is very likely and a positive response to a trial of inhaled or oral steroids is likely |
Table 3 Feno levels as an aid in the management of asthma.
| FeNO (ppb) | Range | Eosinophilic airway inflammation | Interpretation (as an aid in the management of asthma) | |
|---|---|---|---|---|
| Adults | Children | |||
| 510152025 | Low | Unlikely | If symptomatic, review diagnosisNeutrophilic asthmaAnxiety/hyperventilationVocal cord dysfunctionRhinosinusitisGastro‐oesophageal reflux | If symptomatic, review diagnosisConsider also:Wheezy bronchitisCystic fibrosisCongenital abnormalities, e.g. airway malaciaPrimary ciliary dyskinesia |
| If asymptomatic and taking ICS:Implies good compliance with treatment. Reduce dose or, in case of low ICS dose, even withdraw ICS altogether | If asymptomatic and taking ICS: as for adults | |||
| 303540 | Intermediate | Present but mild | If symptomatic, consider:Infection as reason for worseningHigh levels of allergen exposureAdding in other therapy apart from ICS (e.g. long acting β agonist)Consider ICS dose increase | If symptomatic (besides considerations in adults), consider:Possible inadequate ICS treatment(1) check compliance(2) check for poor inhaler technique and consider metered dose inhaler and spacer if patient is currently using a dry powder device |
| If asymptomaticNo change in ICS dose if patient is stable | If asymptomatic: as for adults | |||
| 4550556065Higher levels | High (or rise of 60% or more since previous measurement) | Significant | If symptomatic, consider:Inadequate ICS treatment:(1) check compliance(2) check for poor inhaler technique(3) inadequate ICS doseContinuous high level allergen exposureImminent exacerbation or relapse depending on history of individual patient. More likely if ICS dose is zeroSteroid resistance (rare) | If symptomatic (besides considerations in adults) consider:Metered dose inhaler and spacer if patient is currently using a dry powder device |
| If asymptomaticNo change in ICS dose if patient is stable | If asymptomatic: as for adults | |||
Diagnostic use is fairly straightforward (table 2). For regular monitoring in subjects with chronic asthma (table 3), raised Feno levels in a symptomatic patient indicate uncontrolled eosinophilic airway inflammation. This is most frequently due to poor compliance with anti‐inflammatory treatment or poor inhaler technique rather than inadequate ICS dosing. Although poor inhaler technique resulting in inadequate drug deposition is a plausible reason for raised Feno values, in a study of asthmatic children on a median dose of 800 μg budesonide this could not be proven.139 Where Feno levels remain high despite a seemingly adequate inhaled drug regime, it is theoretically possible that overexpression of constitutive steroid resistant NOS may be the explanation. Alternatively, alveolar NO rather than airway NO may be the source150,151 and, in such circumstances, a better clinical response may be achieved using oral rather than inhaled treatment.152 This remains controversial. Only rarely does a persistently high Feno level indicate true steroid resistance.
A low Feno level implies the absence of eosinophilic airway inflammation and, assuming that the result is not confounded by current tobacco smoking which may reduce Feno levels by up to 60%, an alternative or additional diagnosis to atopic asthma should be considered if the patient is symptomatic. The more common examples include non‐atopic (possibly neutrophilic) asthma, gastro‐oesophageal reflux disease, rhinosinusitis with postnasal drip, and left ventricular dysfunction.
The information contained in tables 2 and 3 is intended for guidance only. Future studies may indicate the need for modifications. Also, as indicated previously, patients with different clinical phenotypes may have different baseline values and different “target” Feno levels may be appropriate. This is because even when asthma is stable, Feno levels may remain high. As a strategy, evidence that “normalising” the Feno results in clinical benefit has not yet been documented. Rather, individualised “Feno typing” and cut points may be required. In some steroid dependent patients with asthma we have found it appropriate to devise a “sliding scale” which relates oral prednisone dose requirements to changes in Feno.
Conclusions
Feno measurements offer a step forward in the assessment of airways disease. As an “inflammometer”, Feno provides the clinician with hitherto unavailable information regarding the nature of underlying airway inflammation, thus complementing conventional physiological testing, including the measurement of AHR. Feno measurements are easy to perform, reproducible, and technically less demanding than induced sputum analysis. They are unreliable in current smokers and, when used diagnostically, in patients who have been taking inhaled or oral steroids recently.
Feno results require careful reference to the clinical context. In symptomatic patients, high Feno levels (>50 ppb) indicate significant airway eosinophilia which is likely to respond to ICS treatment. This appears to be independent of the diagnostic label. Further work is required to confirm how Feno measurements should be interpreted in patients with probable COPD. Present data provide support for the diagnostic use of Feno measurements in children with asthma‐like symptoms, but in the very young more evidence is required. Whether or not Feno may be used to predict steroid response or guide ICS dose requirements in young children with recurrent wheeze is still unclear.
In patients with chronic and/or severe asthma, Feno levels are helpful to determine whether or not eosinophilic airway inflammation is currently active. Both high (>50 ppb) and low (<25 ppb) Feno levels may be used to predict outcomes in patients with a definite history of asthma currently in remission, and in whom withdrawal of ICS therapy is being undertaken. Again, depending on the level of symptoms, both high and low Feno levels offer the clinician information which may help to guide ICS dose adjustment decisions. As yet, however, much more work needs to be done before intermediate values based on group mean data can be used with complete confidence in this setting. The advantages of sequential individual data needs further study.
Abbreviations
AHR - airway hyperresponsiveness
CF - cystic fibrosis
COPD - chronic obstructive pulmonary disease
Feno - exhaled nitric oxide
FEV1 - forced expiratory volume in 1 second
ICS - inhaled corticosteroids
NO - nitric oxide
PCD - primary ciliary dyskinesia
Footnotes
Competing interests: Professor Taylor has received funding from Aerocrine, a manufacturer of nitric oxide analysers.
Note: Unless otherwise stated, all FEno measurements are reported in parts per billion at a flow rate of 50 ml/s. In some instances corrections for flow rate have been made to ensure consistency and permit appropriate interpretation by the reader.
References
- 1.Alving K, Weitzberg E, Lundberg J M. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 199361368–1370. [PubMed] [Google Scholar]
- 2.Ignarro L J. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 200253503–514. [PubMed] [Google Scholar]
- 3.Ricciardolo F L. Multiple roles of nitric oxide in the airways. Thorax 200358175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijkamp F P, Folkerts G. Nitric oxide and bronchial hyperresponsiveness. Arch Int Pharmacodyn Ther 199532981–96. [PubMed] [Google Scholar]
- 5.De Sanctis G T, MacLean J A, Hamada K.et al Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med 19991891621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane C, Knight D, Burgess S.et al Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 200459757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharitonov S A, Barnes P J. Clinical aspects of exhaled nitric oxide. Eur Respir J 200016781–792. [DOI] [PubMed] [Google Scholar]
- 8.Djukanovic R, Roche W R, Wilson J W.et al Mucosal inflammation in asthma. Am Rev Respir Dis 1990142434–457. [DOI] [PubMed] [Google Scholar]
- 9.Jatakanon A, Lim S, Kharitonov S A.et al Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 19985391–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlyne G S, Parameswaran K, Kamada D.et al A comparison of exhaled nitric oxide and induced sputum as markers of airway inflammation. J Allergy Clin Immunol 2000106638–644. [DOI] [PubMed] [Google Scholar]
- 11.Brightling C E, Symon F A, Birring S S.et al Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax 200358528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne D N, Adcock I M, Wilson N M.et al Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 20011641376–1381. [DOI] [PubMed] [Google Scholar]
- 13.van den Toorn L M, Overbeek S E, de Jongste J C.et al Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med 20011642107–2113. [DOI] [PubMed] [Google Scholar]
- 14.Warke T J, Fitch P S, Brown V.et al Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 200257383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strunk R C, Szefler S J, Phillips B R.et al Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol 2003112883–892. [DOI] [PubMed] [Google Scholar]
- 16.Mattes J, Storm van's Gravesande K, Reining U.et al NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid‐dependent childhood asthma. Eur Respir J 1999131391–1395. [PubMed] [Google Scholar]
- 17.Fabbri L M, Romagnoli M, Corbetta L.et al Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003167418–424. [DOI] [PubMed] [Google Scholar]
- 18.Gratziou C, Lignos M, Dassiou M.et al Influence of atopy on exhaled nitric oxide in patients with stable asthma and rhinitis. Eur Respir J 199914897–901. [DOI] [PubMed] [Google Scholar]
- 19.Henriksen A H, Sue‐Chu M, Lingaas Holmen T.et al Exhaled and nasal NO levels in allergic rhinitis: relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur Respir J 199913301–306. [DOI] [PubMed] [Google Scholar]
- 20.Jouaville L F, Annesi‐Maesano I, Nguyen L T.et al Interrelationships among asthma, atopy, rhinitis and exhaled nitric oxide in a population‐based sample of children. Clin Exp Allergy 2003331506–1511. [DOI] [PubMed] [Google Scholar]
- 21.Djukanovic R, Homeyard S, Gratziou C.et al The effect of treatment with oral corticosteroids on asthma symptoms and airway inflammation. Am J Respir Crit Care Med 1997155826–832. [DOI] [PubMed] [Google Scholar]
- 22.Lim S, Jatakanon A, John M.et al Effect of inhaled budesonide on lung function and airway inflammation. Assessment by various inflammatory markers in mild asthma. Am J Respir Crit Care Med 199915922–30. [DOI] [PubMed] [Google Scholar]
- 23.Pavord I D, Brightling C E, Woltmann G.et al Non‐eosinophilic corticosteroid unresponsive asthma. Lancet 19993532213–2214. [DOI] [PubMed] [Google Scholar]
- 24.Green R H, Brightling C E, Woltmann G.et al Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 200257875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzichini E, Pizzichini M M, Gibson P.et al Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med 19981581511–1517. [DOI] [PubMed] [Google Scholar]
- 26.Brightling C E, Monteiro W, Ward R.et al Sputum eosinophilia and short‐term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 20003561480–1485. [DOI] [PubMed] [Google Scholar]
- 27.Little S A, Chalmers G W, MacLeod K J.et al Non‐invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax 200055232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A D, Cowan J O, Brassett K P.et al Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med 2005172453–459. [DOI] [PubMed] [Google Scholar]
- 29.Szefler S J, Phillips B R, Martinez F D.et al Characterization of within‐subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005115233–242. [DOI] [PubMed] [Google Scholar]
- 30.Kharitonov S A, Yates D H, Barnes P J. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med 1996153454–457. [DOI] [PubMed] [Google Scholar]
- 31.Beck‐Ripp J, Griese M, Arenz S.et al Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J 2002191015–1019. [DOI] [PubMed] [Google Scholar]
- 32.Pijnenburg M W, Bakker E M, Hop W C.et al Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med 2005172831–836. [DOI] [PubMed] [Google Scholar]
- 33.Kharitonov S A, Donnelly L E, Montuschi P.et al Dose‐dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax 200257889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones S L, Herbison P, Cowan J O.et al Exhaled NO and assessment of anti‐inflammatory effects of inhaled steroid: dose‐response relationship. Eur Respir J 200220601–608. [DOI] [PubMed] [Google Scholar]
- 35.Silkoff P E, McClean P, Spino M.et al Dose‐response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest 20011191322–1328. [DOI] [PubMed] [Google Scholar]
- 36.Jatakanon A, Kharitonov S, Lim S, Barnes P J. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax 199954108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones S L, Kittelson J, Cowan J O.et al The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med 2001164738–743. [DOI] [PubMed] [Google Scholar]
- 38.Gibson P G, Henry R L, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J 2000161008–1015. [PubMed] [Google Scholar]
- 39.Wenzel S E, Schwartz L B, Langmack E L.et al Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 19991601001–1008. [DOI] [PubMed] [Google Scholar]
- 40.Wenzel S E. A different disease, many diseases or mild asthma gone bad? Challenges of severe asthma. Eur Respir J 200322397–398. [DOI] [PubMed] [Google Scholar]
- 41.Payne D N, Wilson N M, James A.et al Evidence for different subgroups of difficult asthma in children. Thorax 200156345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deykin A, Massaro A F, Drazen J M.et al Exhaled nitric oxide as a diagnostic test for asthma: online versus offline techniques and effect of flow rate. Am J Respir Crit Care Med 20021651597–1601. [DOI] [PubMed] [Google Scholar]
- 43.Kharitonov S A, Yates D, Barnes P J. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur Respir J 19958295–297. [DOI] [PubMed] [Google Scholar]
- 44.de Gouw H W, Grunberg K, Schot R.et al Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur Respir J 199811126–132. [DOI] [PubMed] [Google Scholar]
- 45.Dupont L J, Demedts M G, Verleden G M. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003123751–756. [DOI] [PubMed] [Google Scholar]
- 46.Smith A D, Cowan J O, Filsell S.et al Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med 2004169473–478. [DOI] [PubMed] [Google Scholar]
- 47.Smith A D, Taylor D R. Is exhaled nitric oxide measurement a useful clinical test in asthma? Curr Opin Allergy Clin Immunol 2005549–56. [DOI] [PubMed] [Google Scholar]
- 48.Henriksen A H, Lingaas‐Holmen T, Sue‐Chu M.et al Combined use of exhaled nitric oxide and airway hyperresponsiveness in characterizing asthma in a large population survey. Eur Respir J 200015849–855. [DOI] [PubMed] [Google Scholar]
- 49.Berkman N, Avital A, Breuer R.et al Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax 200560383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatkin J M, Ansarin K, Silkoff P E.et al Exhaled nitric oxide as a noninvasive assessment of chronic cough. Am J Respir Crit Care Med 19991591810–1813. [DOI] [PubMed] [Google Scholar]
- 51.Barnes P J, Woolcock A J. Difficult asthma. Eur Respir J 1998121209–1218. [DOI] [PubMed] [Google Scholar]
- 52.Artlich A, Jonsson B, Bhiladvala M.et al Single breath analysis of endogenous nitric oxide in the newborn. Biol Neonate 20017921–26. [DOI] [PubMed] [Google Scholar]
- 53.Baraldi E, Dario C, Ongaro R.et al Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am J Respir Crit Care Med 19991591284–1288. [DOI] [PubMed] [Google Scholar]
- 54.Baraldi E, Scollo M, Zaramella C.et al A simple flow‐driven method for online measurement of exhaled NO starting at the age of 4 to 5 years. Am J Respir Crit Care Med 20001621828–1832. [DOI] [PubMed] [Google Scholar]
- 55.Daniel P F, Klug B, Valerius N H. Measurement of exhaled nitric oxide in young children during tidal breathing through a facemask. Pediatr Allergy Immunol 200516248–253. [DOI] [PubMed] [Google Scholar]
- 56.Buchvald F, Bisgaard H. FeNO measured at fixed exhalation flow rate during controlled tidal breathing in children from the age of 2 yr. Am J Respir Crit Care Med 2001163699–704. [DOI] [PubMed] [Google Scholar]
- 57.Jobsis Q, Schellekens S L, Kroesbergen A.et al Off‐line sampling of exhaled air for nitric oxide measurement in children: methodological aspects. Eur Respir J 200117898–903. [DOI] [PubMed] [Google Scholar]
- 58.Jobsis Q, Schellekens S L, Kroesbergen A.et al Sampling of exhaled nitric oxide in children: end‐expiratory plateau, balloon and tidal breathing methods compared. Eur Respir J 1999131406–1410. [DOI] [PubMed] [Google Scholar]
- 59.Jobsis Q, Raatgeep H C, Hop W C.et al Controlled low flow off line sampling of exhaled nitric oxide in children. Thorax 200156285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ratjen F, Kavuk I, Gartig S.et al Airway nitric oxide in infants with acute wheezy bronchitis. Pediatr Allergy Immunol 200011230–235. [DOI] [PubMed] [Google Scholar]
- 61.Wildhaber J H, Hall G L, Stick S M. Measurements of exhaled nitric oxide with the single‐breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med 199915974–78. [DOI] [PubMed] [Google Scholar]
- 62.Baraldi E, de Jongste J C. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J 200220223–237. [DOI] [PubMed] [Google Scholar]
- 63.ATS/ERS Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005171912–930. [DOI] [PubMed] [Google Scholar]
- 64.Brussee J E, Smit H A, Kerkhof M.et al Exhaled nitric oxide in 4‐year‐old children: relationship with asthma and atopy. Eur Respir J 200525455–461. [DOI] [PubMed] [Google Scholar]
- 65.Malmberg L P, Pelkonen A S, Haahtela T.et al Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax 200358494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moeller A, Franklin P, Hall G L.et al Inhaled fluticasone dipropionate decreases levels of nitric oxide in recurrenty wheezy infants. Pediatr Pulmonol 200438250–255. [DOI] [PubMed] [Google Scholar]
- 67.Straub D A, Moeller A, Minocchieri S.et al The effect of montelukast on lung function and exhaled nitric oxide in infants with early childhood asthma. Eur Respir J 200525289–294. [DOI] [PubMed] [Google Scholar]
- 68.Straub D A, Minocchieri S, Moeller A.et al The effect of montelukast on exhaled nitric oxide and lung function in asthmatic children 2 to 5 years old. Chest 2005127509–514. [DOI] [PubMed] [Google Scholar]
- 69.Frank T L, Adisesh A, Pickering A C.et al Relationship between exhaled nitric oxide and childhood asthma. Am J Respir Crit Care Med 19981581032–1036. [DOI] [PubMed] [Google Scholar]
- 70.Moody A, Fergusson W, Wells A.et al Increased nitric oxide production in the respiratory tract in asymptomatic Pacific Islanders: an association with skin prick reactivity to house dust mite. J Allergy Clin Immunol 2000105895–899. [DOI] [PubMed] [Google Scholar]
- 71.van Amsterdam J G, Janssen N A, de Meer G.et al The relationship between exhaled nitric oxide and allergic sensitization in a random sample of school children. Clin Exp Allergy 200333187–191. [DOI] [PubMed] [Google Scholar]
- 72.Saito J, Inoue K, Sugawara A.et al Exhaled nitric oxide as a marker of airway inflammation for an epidemiologic study in schoolchildren. J Allergy Clin Immunol 2004114512–516. [DOI] [PubMed] [Google Scholar]
- 73.van den Toorn L M, Prins J B, de Jongste J C.et al Benefit from anti‐inflammatory treatment during clinical remission of atopic asthma. Respir Med 200599779–787. [DOI] [PubMed] [Google Scholar]
- 74.Clini E, Bianchi L, Vitacca M.et al Exhaled nitric oxide and exercise in stable COPD patients. Chest 2000117702–707. [DOI] [PubMed] [Google Scholar]
- 75.Delen F M, Sippel J M, Osborne M L.et al Increased exhaled nitric oxide in chronic bronchitis: comparison with asthma and COPD. Chest 2000117695–701. [DOI] [PubMed] [Google Scholar]
- 76.Zietkowski Z, Kucharewicz I, Bodzenta‐Lukaszyk A. The influence of inhaled corticosteroids on exhaled nitric oxide in stable chronic obstructive pulmonary disease. Respir Med 200599816–824. [DOI] [PubMed] [Google Scholar]
- 77.Brindicci C, Ito K, Resta O.et al Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J 20052652–59. [DOI] [PubMed] [Google Scholar]
- 78.Papi A, Romagnoli M, Baraldo S.et al Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 20001621773–1777. [DOI] [PubMed] [Google Scholar]
- 79.Kharitonov S A, Yates D, Robbins R A.et al Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994343133–135. [DOI] [PubMed] [Google Scholar]
- 80.Kharitonov S A, O'Connor B J, Evans D J.et al Allergen‐induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. Am J Respir Crit Care Med 19951511894–1899. [DOI] [PubMed] [Google Scholar]
- 81.Adisesh L A, Kharitonov S A, Yates D H.et al Exhaled and nasal nitric oxide is increased in laboratory animal allergy. Clin Exp Allergy 199828876–880. [DOI] [PubMed] [Google Scholar]
- 82.Murphy A W, Platts‐Mills T A, Lobo M.et al Respiratory nitric oxide levels in experimental human influenza. Chest 1998114452–456. [DOI] [PubMed] [Google Scholar]
- 83.Cremona G, Higenbottam T W, Mayoral V.et al Elevated exhaled nitric oxide in patients with hepatopulmonary syndrome. Eur Respir J 199581883–1885. [DOI] [PubMed] [Google Scholar]
- 84.Rolla G, Brussino L, Colagrande P.et al Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology 199726842–847. [DOI] [PubMed] [Google Scholar]
- 85.Sogni P, Garnier P, Gadano A.et al Endogenous pulmonary nitric oxide production measured from exhaled air is increased in patients with severe cirrhosis. J Hepatol 199523471–473. [DOI] [PubMed] [Google Scholar]
- 86.Gabbay E, Fisher A J, Small T.et al Exhaled single‐breath nitric oxide measurements are reproducible, repeatable and reflect levels of nitric oxide found in the lower airways. Eur Respir J 199811467–472. [DOI] [PubMed] [Google Scholar]
- 87.Gabbay E, Walters E H, Orsida B.et al Post‐lung transplant bronchiolitis obliterans syndrome (BOS) is characterized by increased exhaled nitric oxide levels and epithelial inducible nitric oxide synthase. Am J Respir Crit Care Med 20001622182–2187. [DOI] [PubMed] [Google Scholar]
- 88.Fisher A J, Gabbay E, Small T.et al Cross sectional study of exhaled nitric oxide levels following lung transplantation. Thorax 199853454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verleden G M, Dupont L J, Van Raemdonck D E.et al Accuracy of exhaled nitric oxide measurements for the diagnosis of bronchiolitis obliterans syndrome after lung transplantation. Transplantation 200478730–733. [DOI] [PubMed] [Google Scholar]
- 90.Brugiere O, Thabut G, Mal H.et al Exhaled NO may predict the decline in lung function in bronchiolitis obliterans syndrome. Eur Respir J 200525813–819. [DOI] [PubMed] [Google Scholar]
- 91.Ho L P, Innes J A, Greening A P. Exhaled nitric oxide is not elevated in the inflammatory airways diseases of cystic fibrosis and bronchiectasis. Eur Respir J 1998121290–1294. [DOI] [PubMed] [Google Scholar]
- 92.Kharitonov S A, Wells A U, O'Connor B J.et al Elevated levels of exhaled nitric oxide in bronchiectasis. Am J Respir Crit Care Med 19951511889–1893. [DOI] [PubMed] [Google Scholar]
- 93.Tsang K W, Leung R, Fung P C.et al Exhaled and sputum nitric oxide in bronchiectasis: correlation with clinical parameters. Chest 200212188–94. [DOI] [PubMed] [Google Scholar]
- 94.Agusti A G, Villaverde J M, Togores B.et al Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur Respir J 199914523–528. [DOI] [PubMed] [Google Scholar]
- 95.Ansarin K, Chatkin J M, Ferreira I M.et al Exhaled nitric oxide in chronic obstructive pulmonary disease: relationship to pulmonary function. Eur Respir J 200117934–938. [DOI] [PubMed] [Google Scholar]
- 96.Clini E, Bianchi L, Ambrosino N. Exhaled nitric oxide in COPD patients. Monaldi Arch Chest Dis 200156169–170. [PubMed] [Google Scholar]
- 97.Clini E, Cremona G, Campana M.et al Production of endogenous nitric oxide in chronic obstructive pulmonary disease and patients with cor pulmonale. Correlates with echo‐Doppler assessment. Am J Respir Crit Care Med 2000162446–450. [DOI] [PubMed] [Google Scholar]
- 98.Corradi M, Majori M, Cacciani G C.et al Increased exhaled nitric oxide in patients with stable chronic obstructive pulmonary disease. Thorax 199954572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanazawa H, Shoji S, Yoshikawa T.et al Increased production of endogenous nitric oxide in patients with bronchial asthma and chronic obstructive pulmonary disease. Clin Exp Allergy 1998281244–1250. [DOI] [PubMed] [Google Scholar]
- 100.Maziak W, Loukides S, Culpitt S.et al Exhaled nitric oxide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998157998–1002. [DOI] [PubMed] [Google Scholar]
- 101.Rutgers S R, van der Mark T W, Coers W.et al Markers of nitric oxide metabolism in sputum and exhaled air are not increased in chronic obstructive pulmonary disease. Thorax 199954576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silkoff P E, Martin D, Pak J.et al Exhaled nitric oxide correlated with induced sputum findings in COPD. Chest 20011191049–1055. [DOI] [PubMed] [Google Scholar]
- 103.Paredi P, Kharitonov S A, Loukides S.et al Exhaled nitric oxide is increased in active fibrosing alveolitis. Chest 19991151352–1356. [DOI] [PubMed] [Google Scholar]
- 104.Moodley Y P, Chetty R, Lalloo U G. Nitric oxide levels in exhaled air and inducible nitric oxide synthase immunolocalization in pulmonary sarcoidosis. Eur Respir J 199914822–827. [DOI] [PubMed] [Google Scholar]
- 105.Rolla G, Colagrande P, Scappaticci E.et al Exhaled nitric oxide in systemic sclerosis: relationships with lung involvement and pulmonary hypertension. J Rheumatol 2000271693–1698. [PubMed] [Google Scholar]
- 106.Moodley Y P, Lalloo U G. Exhaled nitric oxide is elevated in patients with progressive systemic sclerosis without interstitial lung disease. Chest 20011191449–1454. [DOI] [PubMed] [Google Scholar]
- 107.Kharitonov S A, Cailes J B, Black C M.et al Decreased nitric oxide in the exhaled air of patients with systemic sclerosis with pulmonary hypertension. Thorax 1997521051–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grasemann H, Michler E, Wallot M.et al Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatr Pulmonol 199724173–177. [DOI] [PubMed] [Google Scholar]
- 109.Dotsch J, Demirakca S, Terbrack H G.et al Airway nitric oxide in asthmatic children and patients with cystic fibrosis. Eur Respir J 199692537–2540. [DOI] [PubMed] [Google Scholar]
- 110.Elphick H E, Demoncheaux E A, Ritson S.et al Exhaled nitric oxide is reduced in infants with cystic fibrosis. Thorax 200156151–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karadag B, James A J, Gultekin E.et al Nasal and lower airway level of nitric oxide in children with primary ciliary dyskinesia. Eur Respir J 1999131402–1405. [DOI] [PubMed] [Google Scholar]
- 112.Wodehouse T, Kharitonov S A, Mackay I S.et al Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur Respir J 20032143–47. [DOI] [PubMed] [Google Scholar]
- 113.Riley M S, Porszasz J, Miranda J.et al Exhaled nitric oxide during exercise in primary pulmonary hypertension and pulmonary fibrosis. Chest 199711144–50. [DOI] [PubMed] [Google Scholar]
- 114.Loveless M O, Phillips C R, Giraud G D.et al Decreased exhaled nitric oxide in subjects with HIV infection. Thorax 199752185–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brett S J, Evans T W. Measurement of endogenous nitric oxide in the lungs of patients with the acute respiratory distress syndrome. Am J Respir Crit Care Med 1998157993–997. [DOI] [PubMed] [Google Scholar]
- 116.Downey D, Elborn J S. Nitric oxide, iNOS, and inflammation in cystic fibrosis. J Pathol 2000190115–116. [DOI] [PubMed] [Google Scholar]
- 117.Kelley T J, Drumm M L. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest 19981021200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ho L P, Innes J A, Greening A P. Nitrite levels in breath condensate of patients with cystic fibrosis is elevated in contrast to exhaled nitric oxide. Thorax 199853680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grasemann H, Ioannidis I, Tomkiewicz R P, de Groot H, Rubin B K, Ratjen F. Nitric oxide metabolites in cystic fibrosis lung disease. Arch Dis Child 19987849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lundberg J O, Rinder J, Weitzberg E.et al Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand 1994152431–432. [DOI] [PubMed] [Google Scholar]
- 121.Narang I, Ersu R, Wilson N M.et al Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax 200257586–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Corbelli R, Bringolf‐Isler B, Amacher A.et al Nasal nitric oxide measurements to screen children for primary ciliary dyskinesia. Chest 20041261054–1059. [DOI] [PubMed] [Google Scholar]
- 123.Grasemann H, Gartig S S, Wiesemann H G.et al Effect of L‐arginine infusion on airway NO in cystic fibrosis and primary ciliary dyskinesia syndrome. Eur Respir J 199913114–118. [DOI] [PubMed] [Google Scholar]
- 124.Loukides S, Kharitonov S, Wodehouse T.et al Effect of arginine on mucociliary function in primary ciliary dyskinesia. Lancet 1998352371–372. [DOI] [PubMed] [Google Scholar]
- 125.Csoma Z, Bush A, Wilson N M.et al Nitric oxide metabolites are not reduced in exhaled breath condensate of patients with primary ciliary dyskinesia. Chest 2003124633–638. [DOI] [PubMed] [Google Scholar]
- 126.Baraldi E, Pasquale M F, Cangiotti A M.et al Nasal nitric oxide is low early in life: case study of two infants with primary ciliary dyskinesia. Eur Respir J 200424881–883. [DOI] [PubMed] [Google Scholar]
- 127.Jain B, Rubinstein I, Robbins R A.et al Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun 199319183–88. [DOI] [PubMed] [Google Scholar]
- 128.Sanders S P, Proud D, Permutt S.et al Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J Allergy Clin Immunol 2004113697–702. [DOI] [PubMed] [Google Scholar]
- 129.Jatakanon A, Lim S, Barnes P J. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med 200016164–72. [DOI] [PubMed] [Google Scholar]
- 130.Harkins M S, Fiato K L, Iwamoto G K. Exhaled nitric oxide predicts asthma exacerbation. J Asthma 200441471–476. [DOI] [PubMed] [Google Scholar]
- 131.Leuppi J D, Salome C M, Jenkins C R.et al Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am J Respir Crit Care Med 2001163406–412. [DOI] [PubMed] [Google Scholar]
- 132.Deykin A, Lazarus S C, Fahy J V.et al Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol 2005115720–727. [DOI] [PubMed] [Google Scholar]
- 133.Zacharasiewicz A, Wilson N, Lex C.et al Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med 20051711077–1082. [DOI] [PubMed] [Google Scholar]
- 134.Pijnenburg M W, Hofhuis W, Hop W C.et al Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax 200560215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jayaram L, Pizzichini M M, Cook R J.et al Determining asthma treatment by monitoring sputum cell counts: effect on exacerbation. Eur Respir J 200627483–494. [DOI] [PubMed] [Google Scholar]
- 136.Sont J K, Han J, van Krieken J M.et al Relationship between the inflammatory infiltrate in bronchial biopsy specimens and clinical severity of asthma in patients treated with inhaled steroids. Thorax 199651496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Silvestri M, Sabatini F, Sale R.et al Correlations between exhaled nitric oxide levels, blood eosinophilia, and airway obstruction reversibility in childhood asthma are detectable only in atopic individuals. Pediatr Pulmonol 200335358–363. [DOI] [PubMed] [Google Scholar]
- 138.Smith A D, Cowan J O, Brassett K P.et al Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 20053522163–2173. [DOI] [PubMed] [Google Scholar]
- 139.Pijnenburg M W, Bakker E M, Lever S.et al High fractional concentration of nitric oxide in exhaled air despite steroid treatment in asthmatic children. Clin Exp Allergy 200535920–925. [DOI] [PubMed] [Google Scholar]
- 140.Kharitonov S A, Robbins R A, Yates D.et al Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med 1995152609–612. [DOI] [PubMed] [Google Scholar]
- 141.McSharry C P, McKay I C, Chaudhuri R.et al Short and long‐term effects of cigarette smoking independently influence exhaled nitric oxide concentration in asthma. J Allergy Clin Immunol 200511688–93. [DOI] [PubMed] [Google Scholar]
- 142.Robbins R A, Millatmal T, Lassi K.et al Smoking cessation is associated with an increase in exhaled nitric oxide. Chest 1997112313–318. [DOI] [PubMed] [Google Scholar]
- 143.Olin A C, Alving K, Toren K. Exhaled nitric oxide: relation to sensitization and respiratory symptoms. Clin Exp Allergy 200434221–226. [DOI] [PubMed] [Google Scholar]
- 144.van der Lee I, van den Bosch J M, Zanen P. Reduction of variability of exhaled nitric oxide in healthy volunteers. Respir Med 2002961014–1020. [DOI] [PubMed] [Google Scholar]
- 145.Buchvald F, Baraldi E, Carraro S.et al Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol 20051151130–1136. [DOI] [PubMed] [Google Scholar]
- 146.Kharitonov S A, Gonio F, Kelly C.et al Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J 200321433–438. [DOI] [PubMed] [Google Scholar]
- 147.Ekroos H, Tuominen J, Sovijarvi A R. Exhaled nitric oxide and its long‐term variation in healthy non‐smoking subjects. Clin Physiol 200020434–439. [DOI] [PubMed] [Google Scholar]
- 148.Ekroos H, Karjalainen J, Sarna S.et al Short‐term variability of exhaled nitric oxide in young male patients with mild asthma and in healthy subjects. Respir Med 200296895–900. [DOI] [PubMed] [Google Scholar]
- 149.Kissoon N, Duckworth L J, Blake K V.et al Exhaled nitric oxide concentrations: online versus offline values in healthy children. Pediatr Pulmonol 200233283–292. [DOI] [PubMed] [Google Scholar]
- 150.Silkoff P E, Sylvester J T, Zamel N.et al Airway nitric oxide diffusion in asthma: Role in pulmonary function and bronchial responsiveness. Am J Respir Crit Care Med 20001611218–1228. [DOI] [PubMed] [Google Scholar]
- 151.Shin H W, Rose‐Gottron C M, Cooper D M.et al Airway diffusing capacity of nitric oxide and steroid therapy in asthma. J Appl Physiol 20049665–75. [DOI] [PubMed] [Google Scholar]
- 152.Berry M, Hargadon B, Morgan A.et al Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur Respir J 200525986–991. [DOI] [PubMed] [Google Scholar]