Abstract
The treatment of ischemic strokes is limited to prophylactic agents that block the coagulation cascade. Here, we show that cholesterol-lowering agents, 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors, protect against cerebral injury by a previously unidentified mechanism involving the selective up-regulation of endothelial NO synthase (eNOS). Prophylactic treatment with HMG-CoA reductase inhibitors augments cerebral blood flow, reduces cerebral infarct size, and improves neurological function in normocholesterolemic mice. The up-regulation of eNOS by HMG-CoA reductase inhibitors is not associated with changes in serum cholesterol levels, but is reversed by cotreatment with l-mevalonate and by the downstream isoprenoid, geranylgeranyl pyrophosphate and not by farnesyl pyrophosphate. The blood flow and neuroprotective effects of HMG-CoA reductase inhibitors are completely absent in eNOS-deficient mice, indicating that enhanced eNOS activity by HMG-CoA reductase inhibitors is the predominant if not the only mechanism by which these agents protect against cerebral injury. Our results suggest that HMG-CoA reductase inhibitors provide a prophylactic treatment strategy for increasing blood flow and reducing brain injury during cerebral ischemia.
Keywords: cerebral blood flow/cerebral ischemia
Ischemic stroke is the third leading cause of death in the United States and is frequently associated with long-term disability, especially in the elderly population and in patients undergoing cardiovascular surgery (1). Currently, few therapeutic options are available for the treatment of ischemic strokes with prophylactic treatment strategies limited mainly to agents that block platelet aggregation or the coagulation cascade (2). Although clinical trials have demonstrated the effectiveness of antiplatelet agents in decreasing the incidence of ischemic strokes, these agents, however, do not address the larger issue of reducing cerebral infarct size (2).
The 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors are potent inhibitors of cholesterol biosynthesis (3, 4). These drugs, which are also referred to as statins according to their generic names like simvastatin (Sim), lovastatin (Lov), pravastatin, and atorvastatin, are widely prescribed to treat hypercholesterolemic individuals (5–9). Recent large clinical trials demonstrate that statins markedly decrease serum cholesterol levels and reduce the incidence of myocardial and cerebral infarctions (5–8, 10). It is presumed that by lowering serum cholesterol levels, statins may cause regression or stabilization of atherosclerotic plaques (11). However, recent studies have challenged this notion and suggest that the beneficial effects of these agents may extend beyond cholesterol reduction (12–17). For example, meta-analyses of recent lipid-lowering clinical trials suggest that despite comparable reduction in serum cholesterol levels, the risk of myocardial infarctions in the statin-treated group is significantly lower compared with other agents or modalities used to decrease serum cholesterol levels (12–15). Furthermore, treatment with statins improves endothelial function in the absence of significant changes in serum cholesterol levels (15, 16). These results are consistent with recent findings that statins can directly up-regulate endothelial (type III) NO synthase (eNOS) expression in vitro under cholesterol-clamped conditions (18–20).
NO is an important mediator of vascular homeostasis and blood flow (21–25). The loss of endothelial NO impairs vascular function in part by promoting vasoconstriction, platelet aggregation, smooth muscle cell proliferation and leukocyte adhesion (21, 25). Indeed, mice that lack the gene for eNOS are relatively hypertensive and exhibit larger cerebral infarctions after middle cerebral artery (MCA) occlusion (23, 26). Similarly, inhibition of eNOS activity decreases cerebral blood flow (CBF) and promotes tissue damage after focal ischemia (27). In contrast, enhanced NO production by either administration of NO donors or the eNOS substrate, l-arginine, confers stroke protection after induction of cerebral ischemia (28–30).
We hypothesize that HMG-CoA reductase inhibitors modulate eNOS expression and protect against ischemic strokes by mechanisms that are independent of serum cholesterol levels. The purpose of this study, therefore, is to determine whether treatment with HMG-CoA reductase inhibitors can reduce cerebral ischemia and infarct size by up-regulating eNOS expression and activity in normocholesterolemic mice.
METHODS
Drugs.
HMG-CoA reductase inhibiting drugs (Sim and Lov, Merck) were chemically activated by alkaline hydrolysis before subcutaneous injection or cell culture treatment as described (9, 19).
Cell Culture.
Human saphenous vein endothelial cells were cultured as described (18–20). Human neuroblastoma (SH-SY5Y) and pheochromocytoma cell lines (PC-12) were kindly provided by Gloria Lee, Harvard Medical School. Cells were cultured and differentiated by treatment with 16 nM 12-O-tetradecanoylphorbol 13-acetate (Sigma) for SH-SY5Y and with 100 ng/ml of mouse nerve growth factor (Promega) for PC-12 as described (31, 32). Hypoxic conditions and evaluation of survival by trypan blue exclusion were performed as reported (18, 19).
Western Blot Analysis.
Immunoblotting using a murine mAb to human eNOS (Transduction Laboratories, Lexington, KY) was performed as described (18–20).
Drug Treatment.
All animal experiments were conducted in accordance to National Institutes of Health and institutional guidelines. 129/SVEvTacBR mice (18–22 g, Taconic Farms), C57BL/6NCrlBR mice (Charles River Laboratories) and eNOS-deficient mice (23) (18–22 g) were injected s.c. with 0.1 ml of activated Sim or Lov (0.2–20 mg/kg) or a corresponding volume of PBS once daily for 3 or 14 days.
Model of Focal Cerebral Ischemia.
Animals were anesthetized with 1.5% halothane and maintained on 1.0% halothane in 70% N2O and 30% O2 by a face mask. Cerebral infarcts were produced by 2 h of MCA occlusion followed by reperfusion as described (26, 27, 33). To do so, we introduced a silicone-coated 8–0 monofilament in the internal carotid artery and advanced it. After 2 h the animals were briefly reanesthetized and the filament withdrawn. Regional CBF and physiologic parameters were monitored as described (26, 27, 33). In randomly selected animals the left femoral artery was cannulated for arterial blood pressure and blood gas determination. Arterial blood samples were analyzed for pH, arterial oxygen pressure and partial pressure of carbon dioxide by using a blood gas/pH analyzer (Corning 178, CIBA–Corning Diagnostics, Medford, MA). Rectal temperature was maintained at 36.5 ± 1°C with a temperature control unit (FHC, Brunswick, ME) during the monitoring period. In some animals anesthesia was withdrawn after MCA occlusion and normal fluctuations in rectal and temporalis muscle temperature were measured for up to 12 h.
Neurological Deficits.
Animals were tested for neurological deficits [from 0 (no deficit) to 3 (severe)] either 22 or 70 h after reperfusion by an impartial observer as described (33).
Determination of Infarct Size.
After sacrifice, cerebral infarct sizes were determined on 2,3,5-triphenyltetrazolium chloride (TTC)-stained 2-mm brain sections (24 h) or hematoxylin and eosin-stained 20-μm cryostat sections (72 h) by means of an image analysis system (M4, Imaging Research, St. Catherine’s, ON, Canada) as described (26, 27, 33). After 24 h, the TTC-method cannot reliably determine infarct size because of invading white blood cells and microglia.
Determination of Absolute CBF.
Absolute CBF was measured in anesthetized and ventilated animals under resting conditions or 30 min after filamentous MCA occlusion by using an indicator fractionation technique as described (34). For measurements during ischemia, tissue was obtained and values compared from homologous regions (ischemic and contralateral) enriched with tissue in the middle cerebral artery territory.
Laboratory Testing.
Serum cholesterol, glucose, creatinine, creatinine kinase, and transaminases were determined by Tufts Veterinary Diagnostics Laboratory (Grafton, MA).
Semiquantitative Reverse Transcription–PCR.
Tissues (aortas or cerebral hemispheres) were quickly isolated and frozen after sacrifice. Total RNA isolation, reverse transcription, and semiquantitative competitive PCR was performed according to standard techniques. The sense (5′-TTCCGGCTGCCACCTGATCCTAA-3′) and antisense (5′-AACATATGTCCTTGCTCAAGGCA-3′) primers for eNOS amplified a 340-bp fragment of murine eNOS that was confirmed by DNA sequencing (35, 36). Primer pairs for neuronal NOS (nNOS), inducible NOS (iNOS), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as described elsewhere (35–37). Each PCR cycle consisted of denaturing at 94°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 60 s. The linear exponential phases for eNOS and GAPDH PCR were 35 and 22 cycles, respectively. Equal amounts of corresponding NOS and GAPDH reverse transcription–PCR products were loaded on 1.5% agarose gels. Optical densities of ethidium-bromide stained DNA bands were quantitated using the National Institutes of Health image program (19) and results are expressed as NOS/GAPDH ratios.
NOS Catalytic Activity.
Ca2+-dependent NOS catalytic activity was measured by the conversion of [14C]arginine to [14C]citrulline according to standard protocols with modifications as described (23, 35).
Data Analysis.
Data are presented as mean ± SE. Differences between treatment and control groups were compared by paired or unpaired two-tailed Student’s t test or by ANOVA followed by Scheffe test (physiologic parameters) or Bonferroni test (absolute CBF). For comparisons of neurological deficits a nonparametric test was used (Mann–Whitney rank sum U test). P values of <0.05 were considered statistically significant.
RESULTS
Reduction of Ischemic Stroke Size by Statin Administration.
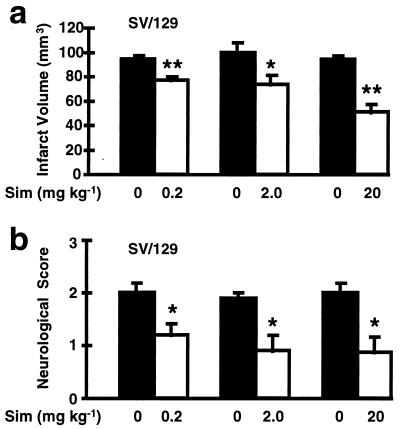
To determine whether statin administration confers protection against ischemic stroke, 129/SV wild-type mice were s.c.-injected daily for 14 days with an HMG-CoA reductase inhibitor Sim (0.2, 2.0 and 20 mg/kg) before MCA occlusion. Cerebral infarct volumes were determined by computer image analysis of TTC-stained 2-mm brain sections. In a concentration-dependent manner, treatment with Sim for 14 days reduced cerebral infarct size by 18, 27 and 46% (Fig. 1A) after 24 h. These results corresponded to decreased neurologic sensory-motor deficits in the Sim treatment groups when evaluated at 24 h (Fig. 1B). The smaller cerebral infarcts after Sim (2 mg/kg, 14 day pretreatment) persisted for at least 3 days (37% reduction in stroke size compared with vehicle after 72 h, n = 8 and 9, P < 0.01). Similar neuroprotective effects of Sim were observed in C57BL/6 mice (32% reduction in infarct size compared with untreated animals after 24 h; P < 0.05, n = 8).
Figure 1.
(A) The effects of Sim (0.2, 2.0 or 20 mg/kg s.c. daily for 14 days) on cerebral infarct volume after 2 h of filamentous MCA occlusion and 22 h of reperfusion compared with vehicle-injected 129/SV mice. Cerebral infarction volume was determined quantitatively by TTC staining as described (31). Smaller infarct sizes were confirmed with an indirect method that corrects for brain swelling (31). (n = 9–18 per group). ∗, P < 0.05; ∗∗, P < 0.01. (B) Neurological sensory-motor deficits were significantly reduced in Sim-treated 129/SV mice compared with controls at 24 h. Deficits were evaluated blindly by an observer using an established rating system ranging from 0 (no deficit) to 3 (severe) (31). (n = 9–18 per group). ∗, P < 0.05.
To evaluate the time-dependent effects of statin administration, animals were pretreated with Sim (2 or 20 mg/kg daily) for a shorter duration before MCA occlusion. Treatment with Sim (20 mg/kg) for 3 days produced less neuroprotective effects compared with that of 14 days (Table 1). To determine if the neuroprotective effects of Sim are shared by other statins, mice were treated with another HMG-CoA reductase inhibitor, Lov (20 mg/kg daily) for 14 days. Lov also decreased cerebral infarct size and neurological deficits, albeit to a lesser extent (Table 1) compared with Sim (Fig. 1). The lower potency of Lov at equimolar concentrations (≈49 μmol/kg) corresponds to the Lov’s higher IC50 value for HMG-CoA reductase compared with that of Sim (38).
Table 1.
Cerebral infarct sizes and neurological deficits following MCA occlusion
| Drug (mg/kg) | Pretreatment, days | Sacrifice, h | Infarct, mm3 | Deficits |
|---|---|---|---|---|
| Vehicle | 3 (n = 5) | 24 | 92 ± 6 | 1.8 ± 0.2 |
| Sim (2.0) | 3 (n = 5) | 24 | 76 ± 10 | 1.2 ± 0.5 |
| Vehicle | 3 (n = 9) | 24 | 103 ± 8 | 1.6 ± 0.3 |
| Sim (20.0) | 3 (n = 10) | 24 | 75 ± 10* | 1.0 ± 0.2 |
| Vehicle | 14 (n = 10) | 24 | 95 ± 5 | 1.9 ± 0.1 |
| Lov (20.0) | 14 (n = 9) | 24 | 76 ± 7* | 1.1 ± 0.3* |
Wild-type SV/129 mice were injected subcutaneously daily with an HMG-CoA reductase inhibitor (Sim or Lov) or vehicle for 3 or 14 days prior to 2 h of MCA occlusion (31). Cerebral infarct volume was determined on TTC-stained brain sections at 24 h. Neurological deficits were rated by an observer naïve to the treatment group or protocol prior to sacrifice. ∗, P < 0.05.
Systemic Physiologic Parameters Are Unaltered after Chronic Statin Administration.
Physiological variables were monitored for each treatment group (Sim: 0.2, 2, and 20 mg/kg daily for 14 days, n = 6 per group) before, during and after MCA occlusion (Table 2). Overall, there were no significant changes in arterial blood pressure, heart rate, glucose, PaCO2, PaO2, pH, or rectal temperature in the treatment groups compared with controls, although treatment with the highest concentration of Sim (20 mg/kg) caused a slight decrease in blood pressure during and a small increase in heart rate after MCA occlusion. These changes, however, are minimal, and most likely, are the result of higher eNOS activity. Rectal and temporalis muscle temperatures did not differ between groups (±<0.3°C; P > 0.05) when measured hourly for up to 12 h after MCA occlusion in vehicle and treated animals (n = 5 per group). The changes in regional CBF (rCBF) were comparable between the control and treatment groups indicating that all of the mice were subjected to a similar degree of ischemia after MCA occlusion relative to their basal CBF (Table 2). In addition, when metabolic variables were examined such as serum glucose, total cholesterol levels, and markers of hepatic and renal function, there was no significant difference between control and treatment groups (Table 3).
Table 2.
Physiologic variables at baseline and during ischemia in SV/129 mice:
| Parameter | Control (n = 6) | Sim (0.2 mg/kg) (n = 6) | Sim (2.0 mg/kg) (n = 6) | Sim (20 mg/kg) (n = 6) |
|---|---|---|---|---|
| MABP, mmHg | ||||
| Baseline | 90.3 ± 4.5 | 84.5 ± 4.4 | 86.3 ± 3.8 | 86.3 ± 4.9 |
| During | 88.8 ± 3.0 | 84.8 ± 2.5 | 89.8 ± 2.6 | 73.2 ± 1.7* |
| After | 82.2 ± 4.1 | 84.0 ± 4.8 | 84.3 ± 1.9 | 76.7 ± 5.0 |
| HR, bpm | ||||
| Baseline | 424 ± 26 | 427 ± 23 | 418 ± 12 | 489 ± 15 |
| During | 438 ± 32 | 416 ± 25 | 469 ± 31 | 488 ± 18 |
| After | 466 ± 17 | 416 ± 25 | 470 ± 23 | 560 ± 9** |
| pH | ||||
| Baseline | 7.31 ± 0.01 | 7.30 ± 0.03 | 7.32 ± 0.01 | 7.31 ± 0.01 |
| After | 7.30 ± 0.02 | 7.28 ± 0.01 | 7.29 ± 0.01 | 7.27 ± 0.02 |
| PaO2, mmHg | ||||
| Baseline | 147 ± 16 | 162 ± 9 | 156 ± 8 | 150 ± 5 |
| After | 139 ± 15 | 145 ± 7 | 149 ± 9 | 134 ± 6 |
| PaCO2, mmHg | ||||
| Baseline | 43.0 ± 1.7 | 40.0 ± 2.7 | 42.3 ± 1.4 | 42.9 ± 1.2 |
| After | 41.5 ± 1.2 | 41.4 ± 0.9 | 40.2 ± 1.3 | 43.1 ± 1.9 |
| rCBF, % | ||||
| During | 17 ± 3 | 16 ± 4 | 14 ± 2 | 15 ± 2 |
| After | 94 ± 8 | 94 ± 7 | 85 ± 1 | 89 ± 4 |
Mice were injected subcutaneously with Sim (0.2, 2.0, or 20 mg/kg) or saline (controls) for 14 days and then subjected to 2-h MCA occlusion followed by reperfusion (31). MABP (mean arterial blood pressure), HR (heart rate, in beats per min), and rCBF were measured at baseline, during MCA occlusion, and until 30 min after reperfusion (31). Fifty microliters of blood was withdrawn twice, before and directly after MCA occlusion for blood gas determination (pH, PaCO2, PaO2). Rectal temperatures were maintained at 36.5°C ± 1°C by a heating blanket and a feedback control unit and did not differ between groups. Data are presented as mean ± SE. ∗, P < 0.05 vs. control, Sim 0.2 and Sim 2.0; ∗∗, P < 0.05 vs. control, Sim 0.2, Sim 2.0, and vs. baseline and during MCA occlusion.
Table 3.
Serum values in SV/129 mice after chronic statin administration
| Parameter | Control (n = 12) | Sim (2.0 mg/kg) (n = 8) | Sim (20 mg/kg) (n = 7) |
|---|---|---|---|
| Cholesterol, mg/dl | 140 ± 7 | 158 ± 5 | 120 ± 20 |
| ALT, units/liter | 119 ± 29 | 136 ± 45 | 124 ± 27 |
| Creatinine, mg/dl | <0.2 | <0.2 | <0.2 |
| Glucose, mg/dl | 128 ± 2 | n.d. | 124 ± 13 |
Mice were injected subcutaneously with Sim (2.0 or 20 mg/kg) or saline (controls) daily for 14 days. Blood was withdrawn and serum levels of cholesterol, alanine aminotransferase (ALT), creatinine, and glucose were determined. There were no statistically significant differences between groups.
Chronic Statin Administration Augments Absolute CBF.
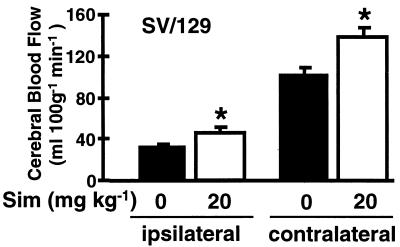
To determine whether the stroke protective effects of Sim were due to a vascular effect, absolute CBF was measured in ventilated mice by using an indicator fractionation technique with N-isopropyl-p-iodo[methyl-1,3-14C]amphetamine (34). Treatment with Sim (20 mg/kg) increased basal hemispheric CBF by 31% compared with controls (151 ± 5 vs. 117 ± 7 ml/100 g per min, P < 0.05, n = 8 per group). Similar increases in resting blood flow were observed in cerebellum and brainstem. Again, when rCBF was measured by using laser-Doppler flowmetry, equivalent percent reductions in rCBF were observed in the treatment group compared with controls during MCA occlusion (Table 2). However, because the resting (baseline) CBF values were higher in Sim-treated animals, there was a correspondingly higher absolute CBF in Sim-treated mice during MCA occlusion. Absolute CBF was 48% and 34% higher in the ischemic and nonischemic hemispheres of Sim-treated mice (20 mg/kg for 14 days), respectively (Fig. 2). Sim had no direct cytoprotective effects on neuronal cell survival and did not protect human neuroblastoma (SH-SY5Y) (31) or rat pheochromocytoma cell lines (PC-12) (32) from hypoxic injury (data not shown) emphasizing the potential importance of vascular mechanisms.
Figure 2.
Effects of Sim on absolute CBF during focal cerebral ischemia in anesthetized and ventilated wild-type 129/SV mice. Mice were injected s.c. with saline (n = 10) or Sim 20 mg/kg (n = 10) for 14 days. Absolute CBF was determined 30 min after filamentous occlusion of the left MCA in the ischemic tissue (ipsilateral) and corresponding tissue from the contralateral hemisphere by using an indicator fractionation technique (32). ∗, P < 0.05.
HMG-CoA Reductase Inhibitors Up-Regulate eNOS.
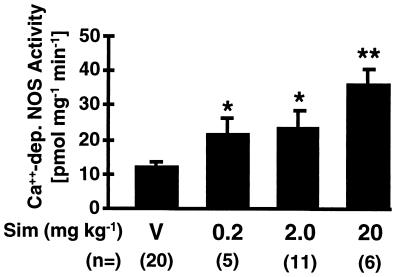
Because eNOS is an important regulator of CBF, we postulate that HMG-CoA reductase inhibitors may augment CBF and confer stroke protection by up-regulating eNOS expression and activity. The eNOS activity was determined by Ca2+-dependent NOS catalytic activity by using the [14C]arginine to [14C]citrulline conversion assay. In a concentration-dependent manner, treatment with Sim for 14 days increased Ca2+-dependent NOS activity in murine aortas by 1.8- to 3.0-fold (Fig. 3). Because of the presence of nNOS, it is not possible to determine eNOS activity in the brain by using the Ca2+-dependent [14C]citrulline assay.
Figure 3.
The Ca2+-dependent NOS catalytic activity was measured in aortas from wild-type 129/SV mice treated with Sim (0.2, 2.0 or 20 mg/kg s.c. for 14 days) compared with vehicle and determined by the conversion of [14C]arginine to [14C]citrulline in vitro. ∗, P < 0.05; ∗∗, P < 0.01.
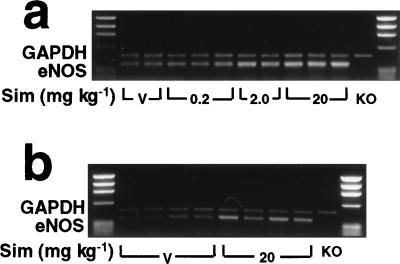
To determine whether increases in NOS activity correlated with increases in eNOS expression, we performed semiquantitative reverse transcription–PCR (on aortas and brains from control and Sim-treated mice). In a concentration-dependent manner, treatment with Sim increased eNOS mRNA levels in the aortas and also in brains of Sim-treated mice (Fig. 4 A and B). However, treatment with Sim did not alter the expression of neuronal NOS in the brain and little or no neuronal NOS was detected in the aorta (not shown). Inducible NOS was not detected under our experimental conditions (not shown).
Figure 4.
Semiquantitative reverse transcription–PCR showing the effects of Sim on eNOS mRNA expression in aortas (a) and brains (b) of 129/SV mice after 14 days of treatment. Specificity was determined by the lack of eNOS mRNA in eNOS-deficient mice (KO). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression did not change with treatment and was used as an internal standard (n = 5 per group).
Inhibition of Geranylgeraniol but Not Cholesterol Synthesis Mediates eNOS Up-Regulation.
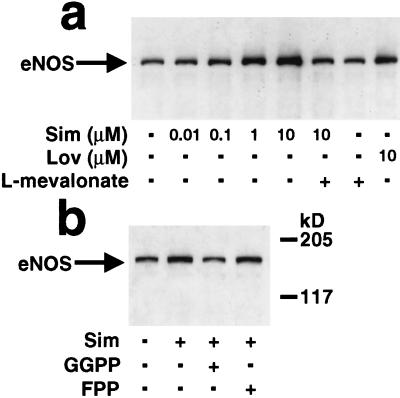
The stroke protective effects of Sim occurred in normocholesterolemic mice and in the absence of significant changes in serum cholesterol levels (Table 3). The up-regulation of eNOS expression in vitro was completely reversed in the presence of l-mevalonate indicating that increases in eNOS expression are mediated via inhibition of endothelial rather than hepatic HMG-CoA reductase (Fig. 5A). To determine which downstream isoprenoid intermediate(s) regulates eNOS expression, endothelial cells were treated with Sim (1 μM) in the presence and absence of geranylgeranyl pyrophosphate (5 μM) or farnesyl pyrophosphate (5 μM). Increase in eNOS protein expression by Sim was not altered by farnesyl pyrophosphate but was completely reversed by geranylgeranyl pyrophosphate, suggesting that inhibition of geranylgeraniol synthesis mediates eNOS up-regulation (Fig. 5B.)
Figure 5.
Immunoblots (20 μg protein/lane) showing the concentration-dependent effects of Sim and Lov on eNOS protein levels in human endothelial cells in the presence and absence of l-mevalonate (200 μM) (17, 18) (a) and in the presence and absence of farnesyl pyrophosphate (FPP, 5 μM) and geranylgeranyl pyrophosphate (GGPP, 5 μM) (b).
No Stroke Protection in eNOS-Deficient Mice Treated with Statins.
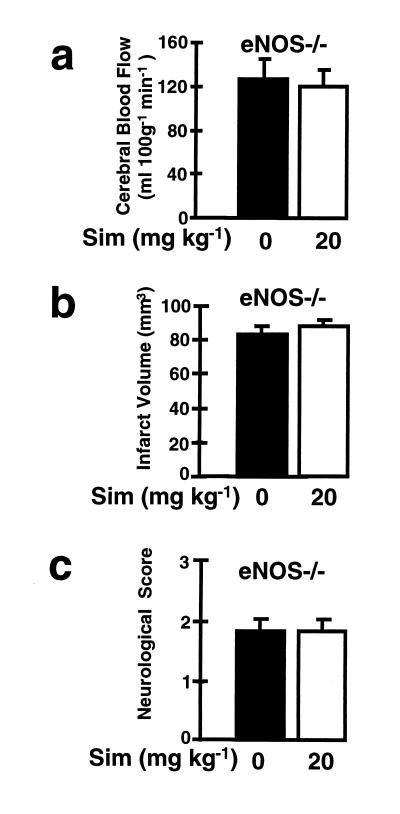
To test our hypothesis that eNOS mediates the stroke protective effects of Sim, we performed similar experiments in eNOS-deficient mice. The background of eNOS-deficient mice is a mixture of 129/SV and C57BL/6 transgenic strains. Compared with 129/SV or C57BL/6 wild-type mice, treatment with Sim had no effect on CBF, infarct size, or neurological deficits in eNOS-deficient mice (Figs. 6). Furthermore, the percent reduction in rCBF during ischemia was not different in Sim- and vehicle-injected eNOS-deficient mice (14 ± 2% and 15 ± 3% of baseline, P > 0.05) and serum cholesterol levels in eNOS-deficient mice were not significantly affected by treatment with Sim (120 ± 20 vs. 140 ± 7 mg/dl, P > 0.05). Again, eNOS-deficient mice have similar levels of neuronal NOS as wild-type mice and no detectable iNOS expression.
Figure 6.
Effects of Sim (Sim, 20 mg/kg daily for 14 days) on (A) absolute CBF in eNOS-deficient mice (eNOS−/−) and (B) cerebral infarct volume and (C ) neurological deficits in eNOS-deficient mice (eNOS−/−) after MCA occlusion/reperfusion (n = 10 per group).
DISCUSSION
The major finding in this study is that prophylactic treatment with HMG-CoA reductase inhibitors protects against ischemic strokes after focal brain ischemia. This is in contrast to previous clinical studies that demonstrate decreases in the incidence but not the size of ischemic strokes due to reductions in serum cholesterol levels (5, 6). Indeed, we find that under our treatment conditions, HMG-CoA reductase inhibitors up-regulate eNOS expression and activity in the absence of significant changes in serum cholesterol levels. The up-regulation of eNOS by HMG-CoA reductase inhibitors leads to an increase in CBF, smaller cerebral infarcts, and decreased neurological deficits in wild-type mice. The mechanism of protection may relate to augmented blood flow but may also stem from other known NO-mediated effects such as inhibition of platelet aggregation or leukocyte adhesion.
The reduction in cerebral infarct size in statin-treated mice was maintained for up to 72 h after MCA occlusion indicating that the beneficial effects of these agents were not transitory. Treatment with statins did not significantly affect physiological parameters such as systemic blood pressure, heart rate, temperature, or blood gases or metabolic variables such as serum glucose or cholesterol levels. Furthermore, the levels of nNOS and iNOS mRNA were not different in wild-type and eNOS-deficient mice and were not changed by subsequent statin treatment. The stroke protective effects of increased vascular NO production are consistent with prior studies demonstrating that intravenous administration of the NO precursor, l-arginine, or an NO donor augments CBF and decreases cerebral ischemia after MCA occlusion in rats (28–30). In fact, Sim-induced augmented CBF values exceeded a critical threshold for CBF in the occluded MCA territory associated previously with a measure of functional recovery (electrocorticogram activity) (28).
The beneficial effects of HMG-CoA reductase inhibitors, however, were absent in eNOS-deficient mice indicating that most if not all beneficial effects are mediated by eNOS. Although infarction volumes were smaller than predicted in eNOS knockout mice, they were large enough to measure a protective effect if present. In fact, infarct volumes were in the range of values reported previously in wild type mice (39) and considerably larger than values obtained in this model after protection with Sim and other treatments such as MK-801 (40), caspase inhibitors (41), or poly(ADP-ribose) polymerase inhibitors (33).
Because many ischemic strokes occur in individuals with average cholesterol levels who may not otherwise be taking statin therapy (8, 10), the up-regulation of eNOS by HMG-CoA reductase inhibitors may be an effective prophylactic treatment strategy for limiting the severity of ischemic strokes in high risk individuals such as patients with a prior history of stroke or transient ischemic attacks. These agents may also be useful for patients undergoing elective cardiovascular procedures that have high perioperative risk for cerebral ischemia such as coronary artery bypass graft surgery or carotid endarterectomy (42). Because HMG-CoA reductase inhibitors increase CBF, these agents may also serve as a useful adjunctive therapeutic modality for increasing the delivery of other coadministered drugs to the central nervous system. Finally, because eNOS expression is up-regulated in the systemic vasculature, HMG-CoA reductase inhibitors may also have beneficial effects in other cardiovascular conditions where endothelial dysfunction may cause or worsen the pathological process such as in atherosclerosis (14–17), pulmonary hypertension (43), and congestive heart failure (44).
The mechanism by which HMG-CoA reductase inhibitors increase eNOS expression and activity occurs via stabilization of eNOS mRNA rather than induction of eNOS gene transcription (19, 20). Furthermore, we have identified the isoprenoid intermediate, geranylgeraniol, the synthesis of which, negatively regulates eNOS expression. These findings are consistent with the cholesterol-independent effect of HMG-CoA reductase inhibitors on eNOS expression and endothelial function.
Because HMG-CoA reductase inhibitors decrease cerebral infarct size in the absence of significant changes in serum cholesterol levels, our findings are distinct from the results of large clinical trials where these agents are found to lower serum cholesterol levels and reduce the incidence of ischemic strokes (5–8). We speculate that HMG-CoA reductase inhibitors may have contributed to the decrease in the incidence of ischemic strokes, in part, by reducing cerebral infarct size to levels that are clinically unappreciated. Based upon our findings, we propose that prophylactic treatment with HMG-CoA reductase inhibitors can decrease the severity of cerebral ischemic damage irrespective of serum cholesterol levels.
Acknowledgments
We thank Christian Waeber for assistance in the NOS activity assay. This work was supported by the National Institutes of Health (grants NS10828 and HL52233 to M.A.M. and J.K.L., respectively) and the Deutsche Forschungsgemeinschaft (En343/1-1 and La1057/1-1 to M.E. and U.L., respectively).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: HMG, 3-hydroxy-3-methylglutaryl; statins, HMG-CoA reductase inhibitors; CBF, cerebral blood flow; rCBF, regional CBF; NOS, NO synthase; eNOS, endothelial NOS; Sim, simvastatin; Lov, lovastatin; MCA, middle cerebral artery; TTC, triphenyltetrazolium chloride; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- 1.Division of Chronic Disease Control and Community. Cardiovascular Disease Surveillance: Stroke, 1980–1989. Centers for Disease Control and Prevention: Atlanta; 1994. [Google Scholar]
- 2.Patrono C. N Engl J Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein J L, Brown M S. Nature (London) 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 4.Massy Z A, Keane W F, Kasiske B L. Lancet. 1996;347:102–103. doi: 10.1016/s0140-6736(96)90217-2. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 6.Scandinavian Simvastatin Survival Study Group. Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 7.Shepherd J, Cobbe S M, Ford I, Isles C G, Lorimer A R, MacFarlane P W, McKillop J H, Packard C J. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 8.Blauw G J, Lagaay A M, Smelt A H, Westendorp R G. Stroke. 1997;28:946–950. doi: 10.1161/01.str.28.5.946. [DOI] [PubMed] [Google Scholar]
- 9.Gerson R J, MacDonald J, Alberts A W, Kornbrust J, Majka J A, Stubbs J, Bokelman D L. Am J Med. 1989;87:28–38. doi: 10.1016/s0002-9343(89)80596-0. [DOI] [PubMed] [Google Scholar]
- 10.Hebert P R, Gaziano J M, Chan K S, Hennekens C H. J Am Med Assoc. 1997;27:313–321. [PubMed] [Google Scholar]
- 11.Libby P. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 12.Packard C J. Circulation. 1998;97:1440–1445. [Google Scholar]
- 13.Buchwald H, Varco R L, Matts J P, Long J M, Fitch L L, Campbell G S, Pearce M B, Yellin A E, Edmiston W A, Smik R D., Jr N Engl J Med. 1990;323:946–955. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- 14.Anderson T J, Meredith I T, Yeung A C, Frei B, Selwyn A P, Ganz P. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 15.O‘Driscoll G, Green D, Taylor R R. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 16.Williams J K, Sukhova G K, Herrington D M, Libby P. J Am Coll Cardiol. 1998;31:684–691. doi: 10.1016/s0735-1097(97)00537-8. [DOI] [PubMed] [Google Scholar]
- 17.Treasure C B, Klein J L, Weintraub W S, Talley J D, Stillabower M E, Kosinski A S, Zhang J, Boccuzzi S J, Cedarholm J C, Alexander R W. N Engl J Med. 1995;334:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 18.Liao J K, Zulueta J J, Feng-Sheng Y, Peng H, Cote C G, Hassoun P M. J Clin Invest. 1995;96:2661–2666. doi: 10.1172/JCI118332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufs U, La Fata V, Liao J K. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 20.Laufs U, La Fata V, Plutzky J, Liao J K. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 21.Palmer R M J, Ferrige A G, Moncada S. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 22.Radomski M W, Palmer R M, Moncada S. Proc Natl Acad Sci USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang P L, Huang Z, Mashimo H, Bloch K D, Moskowitz M A, Bevan J A, Fishman M C. Nature (London) 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 24.Furchgott R F, Zawadzki J V. Nature (London) 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 25.Ignarro L J. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 26.Huang Z, Huang P L, Ma J, Meng W, Ayata C, Fishman M C, Moskowitz M A. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 28.Dalkara T, Morikawa E, Panahian N, Moskowitz M A. Am J Physiol. 1994;267:H678–H683. doi: 10.1152/ajpheart.1994.267.2.H678. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Iadecola C. J Cereb Blood Flow Metab. 1994;14:574–580. doi: 10.1038/jcbfm.1994.71. [DOI] [PubMed] [Google Scholar]
- 30.Morikawa E, Huang Z, Moskowitz M A. Am J Physiol. 1992;263:H1632–H1635. doi: 10.1152/ajpheart.1992.263.5.H1632. [DOI] [PubMed] [Google Scholar]
- 31.Pahlman S, Odelstad L, Larson E, Grotte G, Nilsson K. Int J Cancer. 1981;28:583–589. doi: 10.1002/ijc.2910280509. [DOI] [PubMed] [Google Scholar]
- 32.Brandt R, Leger J, Lee G. J Cell Biol. 1995;131:1327–40. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endres M, Wang Z-Q, Namura S, Waeber C, Moskowitz M A. J Cereb Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Fujii M, Hara H, Meng W, Vonsattel J P, Huang Z, Moskowitz M A. Stroke (Dallas) 1997;28:1805–1811. doi: 10.1161/01.str.28.9.1805. [DOI] [PubMed] [Google Scholar]
- 35.Hara H, Ayata C, Huang P L, Waeber C, Ayata G, Fujii M, Moskowitz M A. J Cereb Blood Flow Metab. 1997;17:515–526. doi: 10.1097/00004647-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Nadaud S, Philippe M, Arnal J-F, Michel J-B, Soubrier F. Circ Res. 1996;79:857–863. doi: 10.1161/01.res.79.4.857. [DOI] [PubMed] [Google Scholar]
- 37.Haddad E K, Duclos A J, Lapp W S, Baines M G. J Immunol. 1997;158:4886–4892. [PubMed] [Google Scholar]
- 38.Van Vliet A K, Negre-Aminou P, van Thiel G C, Bolhuis P A, Cohen L H. Biochem Pharmacol. 1996;52:1387–1392. doi: 10.1016/s0006-2952(96)00467-4. [DOI] [PubMed] [Google Scholar]
- 39.Hara H, Huang P L, Panahian N, Fishman M C, Moskowitz J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Ma, J., Endres, M. & Moskowitz, M. A. (1998) Br. J. Pharmacol., in press.
- 41.Hara H, Friedlander R M, Gagliardini V, Ayata C, Fink K B, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz M A. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sila C A. Neurol Clin. 1998;16:9–20. doi: 10.1016/s0733-8619(05)70364-9. [DOI] [PubMed] [Google Scholar]
- 43.Giaid A, Saleh D. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 44.Katz S D. Curr Opin Cardiol. 1997;12:259–264. doi: 10.1097/00001573-199705000-00007. [DOI] [PubMed] [Google Scholar]