Abstract
The natural history of asthma involves relatively stable periods that are often punctuated by significant exacerbations of symptoms. There are many aetiologies that may lead to an increase in asthma severity including respiratory infection (bacterial/viral), allergens, irritants, and occupational exposures. Each trigger probably acts through different mechanisms, but a final common pathway of multicellular inflammation, enhanced bronchial responsiveness, and airflow obstruction is a likely consequence. This review discusses the most common causes of asthma exacerbations with a focus on their microbiology and immunopathogenesis. Through an understanding of underlying causes of asthma exacerbations, treatments with increased effectiveness may be developed, and it is these future developments that may directly influence the morbidity and mortality of the disease.
Keywords: asthma, exacerbation, aetiology, virus, bacteria
Asthma is a heterogeneous disease that ultimately leads to the clinical constellation of cough, wheeze, and shortness of breath. These symptoms are accompanied by an influx of inflammatory cells. When exacerbations of asthma occur, these underlying clinical features are accentuated and a deterioration of asthma control follows, often despite existing treatment. Many factors contribute to an increase in asthma symptoms including respiratory infections, allergens, irritants, and occupational exposures. Each of these exacerbating factors probably acts through different mechanisms, but has a final common pathway that includes cellular inflammation, enhanced bronchial responsiveness, and greater airflow obstruction.1
Given the importance of asthma exacerbations to a loss of disease control and patient risks for increased morbidity and mortality, it is important to understand and appreciate both the immunopathogenesis of these events and mechanisms leading to increases in asthma severity.
Pathology of asthma
Persistent airway inflammation is a characteristic feature of the disease and includes infiltration of the airways by inflammatory cells (particularly eosinophils), hypertrophy of the smooth muscle, and thickening of the lamina reticularis. There is often disruption of the epithelium. Goblet cell hyperplasia and hypertrophy frequently accompany the loss of epithelial cells and contribute to a narrowing of the airway lumen. The increase in airway muscle also results in a greater narrowing of airway calibre with the same amount of contraction. During exacerbations these airway narrowing processes are accentuated, but it is not clear how these acute events contribute to these underlying changes and the mechanisms underlying an increase in airflow obstruction are not fully understood. Finally, mucus plugs composed of mucus, serum proteins, inflammatory cells, and cellular debris can occlude the airway lumen, but this is typically found in more severe exacerbations.
It is known, however, that asthma exacerbations are associated with both inflammatory and immunological cell infiltration and, presumably, their activation. Although variable in intensity and dependent upon the exacerbating event, the inflammatory cell infiltrate can be multicellular and composed of varying amounts of eosinophils, neutrophils, and lymphocytes. Alternatively, a predominance of one cell type may occur, such as neutrophils with viral infection. T cells in the lung appear to orchestrate an immune response with a strong T helper type 2 (Th2) component when allergens drive the process. Whether this shift in balance towards Th2 reflects diminished Th1 activity and increased susceptibility for infectious events and subsequent exacerbations has not been determined. Epithelial damage may also occur as the result of the inciting event (such as infection) as well as from the ensuing inflammation. Collectively, these events may lead to a cycle of escalating damage and inflammation; the pathway is often determined by the provoking factor—for example, allergens leading to an eosinophilic infiltrate or respiratory viruses that cause a neutrophilic infiltration. Regulation of—or, perhaps, more accurately—a dysregulation of these processes is not well defined but may involve a number of processes including myofibroblasts or T regulatory cells.1
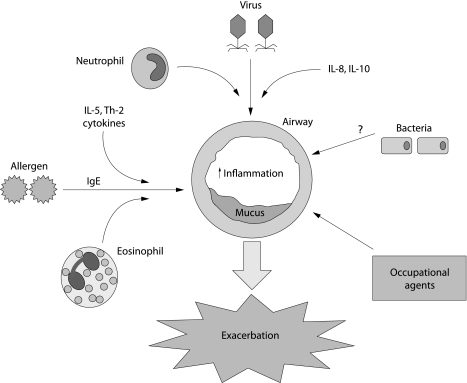
Recent work has provided further insight into the immunopathogenesis of exacerbations at the cellular/molecular level. For example, virus induced exacerbations are characterised by neutrophilic infiltration and, in some cases, eosinophilic degranulation.2 In contrast, allergen induced asthma may stimulate interleukin (IL)‐5 mediated processes as well as other events to attract eosinophils. This is illustrated in fig 1. The following discussion will focus on the most common causes of asthma exacerbations and link the information to current knowledge of asthma in general (see table 1).
Figure 1 Triggers of exacerbation. Multiple aetiologies of asthma exacerbation are shown schematically. Virus induced exacerbation results in a neutrophilic infiltrate with increases in interleukin (IL)‐8 and IL‐10, among other cytokines. Allergen induced exacerbation results in an eosinophilic infiltrate with increases in IL‐5, other Th2 cytokines, and IgE. The mechanisms for bacteria induced exacerbation are not known.
Table 1 Common aetiologies of asthma exacerbations.
| Virus induced | Rhinovirus (RV) |
| Respiratory syncytial virus (RSV) | |
| Human metapneumovirus (HMV) | |
| Influenza virus | |
| Bacteria induced | Mycoplasma pneumoniae |
| Chlamydia pneumoniae | |
| Allergen | Fungi |
| Tree, weed and grass pollen | |
| Indoor allergens | |
| Occupational | Animal exposures |
| Chemical exposures | |
| Irritants | Airway pollutants |
Respiratory virus induced asthma exacerbations
Rhinovirus infections
Respiratory viruses are the most frequent cause of both the common cold and asthma exacerbations. In a study of children aged 9–11 years, 80–85% of asthma exacerbations that resulted in reduced peak expiratory flow and wheezing were due to viral upper respiratory infections.3 Similarly, Nicholson et al4 found that 57% of adult asthma exacerbations were due to upper respiratory tract infections. Of the infections associated with virus induced exacerbations, rhinovirus (RV) was the most commonly found micro‐organism (∼65% of all cases). In addition, admission rates for asthma exacerbations correlate significantly with the seasonal pattern of RV infections (for example, September through December and April).5 RV is thus the major cause of asthma exacerbations and respiratory infection provoked asthma attacks in both children and adults.
RV infections are the major cause of the common cold. Although it was initially felt that RV infections were confined to the upper airway (the site of optimal virus replication (33°C)), it is now accepted that RV can infect the lower airway as well.6 It is proposed that worsening of asthma symptoms arises secondarily to the extension of infection to the lower airway where inflammation increases and airflow obstruction follows.
Microbiology
RV is a small single stranded RNA virus with a capsid of four distinct proteins. There are more than 100 serotypes of RV, most of which bind to intercellular adhesion molecule (ICAM)‐1. Epithelial cells are the principal target of RV, the site of viral replication, and the initiators of the immune/inflammatory response. When RV infects epithelial cells, cytokines and other mediators are released (see below) to recruit cells to the airway. In the acute phase of a cold, neutrophils are the primary inflammatory infiltrate. Plasma leakage also occurs to contribute to airway oedema and a build up of airway secretions. Although not fully established, airway obstruction with virus provoked asthma probably arises from inflammatory cell infiltration, oedema, secretions, and mucus hypersecretion. Other factors, including direct neuronal stimulation by the virus, may also promote bronchospasm and airflow obstruction.
Earlier concepts of this process suggested that RV did not persist and replicate in the lower airway. However, little difference in virus replication was observed when wild‐type RV isolates were incubated at 33°C (upper airway) or 37°C (lower airway). In addition, RV appeared to infect both upper and lower segments of the respiratory tree equally well,7 and the frequency of lower airway infection was similar to that observed in the upper airway.8
Immunopathogenesis
Although cold symptoms usually last 1 week or less, decreases in peak flow with RV infections can persist for a median of 2 weeks in school aged children.3 This association suggests that RV infection persists in the lower airway in asthmatic patients, or suggests that the consequences of an RV infection (such as lower airway features of asthma) last beyond the initial phase of the infection.9
As the RV infection becomes established in the airway epithelium, the virus infected tissue releases a host of cytokines and mediators that probably serve to increase or enhance airway inflammation. It is most likely that the generation of mediators, rather than direct airway injury by RV itself, causes the development of airway inflammation. Increased transcription of the genes encoding many cytokines and chemokines occurs with RV infection, including IL‐1β, IL‐6, IL‐8, IL‐10, IL‐16, granulocyte colony stimulating factor (G‐CSF), interferon (IFN)‐γ and RANTES6,10 both in vitro and in vivo. In addition, Papadopoulos et al11 found an increase in the Th2 cytokines IL‐4 and IL‐13 in asthmatic subjects. Induction of inflammatory cell recruitment and activation may occur as a result of these mediators. RV infections, like other viral infections, cause a transient increase in number of circulating neutrophils that corresponds with symptoms of the upper respiratory infection.8 These cytokines can also increase synthesis of leucocytes to further enhance recruitment to the airway and, perhaps, activate neutrophils to cause inflammation.
Studies of the role of IL‐10 in virus induced exacerbations have shown contrasting results. Grissell and colleagues2 completed a study of 59 patients over the age of 7 years who were admitted to hospital for acute asthma. The group was divided into those with and without a viral infection. Patients with viral infection (RV was again the predominant infection) and an asthma exacerbation had significant increases in IL‐10 and RANTES. The authors proposed that the generation of these factors may explain some of the characteristics of inflammation in virus induced asthma. For example, IL‐10 can suppress eosinophil cellular infiltration whereas RANTES promotes eosinophil degranulation and neutrophil influx. After recovery from the viral illness and exacerbation, IL‐10 levels returned to baseline and sputum eosinophilia reappeared. These observations led the authors to suggest that virus induced asthma has immune responsive features distinct from allergen provoked asthma, and that IL‐10 probably has a unique role in this process.2
In contrast, Corne and colleagues10 measured cytokine, chemokine, and mediator levels in nasal lavage from 44 adults (23 of whom were atopic) taken during the acute and convalescent phases of the common cold. In the acute phase of illness, IL‐10 levels were significantly higher in the non‐atopic group than in the atopic group. There was no significant difference in IL‐10 levels during the convalescent phase. As IL‐10 has been shown to inhibit some proinflammatory cytokines, it was hypothesised that increased IL‐10 levels during the acute phase of infection downregulated and limited the virus induced inflammatory response.10 According to these authors, prolonged inflammatory responses occur as a direct result of this failure to mount an appropriate IL‐10 response in atopic subjects.
Asthma is also associated with an increase in ICAM‐1 expression, a principal receptor for the majority of RV. Thus, upregulation and activation of ICAM‐1, which occurs both with chronic allergen exposure and with RV infection, may explain an increased susceptibility of asthma patients to RV infection.
In addition, recent work has shown that the innate immune response to infection with RV may be restricted and deficient in asthmatic patients.12 Wark and colleagues found that bronchial epithelial cells obtained from asthmatic patients, which were then infected with RV‐16, were resistant to early apoptosis and had a profoundly deficient type I interferon response compared with infected control cells from non‐asthmatic patients. Although there was no difference in expression of ICAM‐1, IL‐6, or RANTES, there was increased viral RNA expression and late cell lysis in the airway tissues from asthmatic patients. The RV infected cells from asthmatic patients lived longer and had decreased apoptosis. Because of these immune defects in host defence, asthmatic patients may be more likely to develop a persistent lower airway infection.
As a result of these findings, the authors investigated whether IFN‐β may have an underlying role in the abnormal antiviral response in asthma. Interestingly, data confirmed that IFN‐β induction by RV infection of bronchial epithelial cells from asthmatic subjects was profoundly impaired and related to decreased apoptosis of epithelial cells and increased viral replication.12 Thus, asthmatic patients may have decreased innate immunity to RV that results in increased cell lysis (causing a more defective epithelial layer and inflammation) and viral replication, but decreased infected cell apoptosis. A defect in IFN‐β may be responsible for these effects.
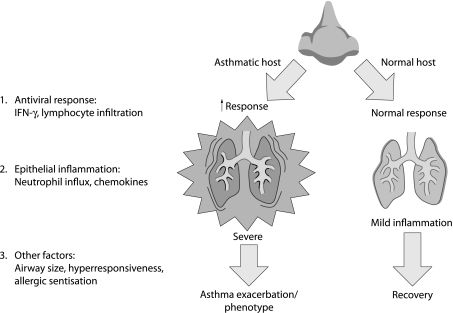
Parry et al13 and Gern et al14 found that peak RV shedding and symptom severity were inversely proportional to IFN‐γ production and directly proportional to IL‐5 production by peripheral blood mononuclear cells. Thus, asthma patients with an enhanced Th1 response were more effective at clearing the illness and vice versa. Clearly, current data suggest that some asthmatic subjects have a dysfunctional antiviral response to RV, and this abnormality may make them more susceptible to the consequences of an infection. Possible host susceptibility factors to viral infection are illustrated in fig 2.
Figure 2 Host susceptibility factors to viral infection. The antiviral response of interferon γ (IFN‐γ) and lymphocyte infiltration results in more rigorous epithelial inflammation in the asthmatic host. An increase in chemokines and neutrophil influx in combination with other factors gives rise to the asthmatic phenotype.
Effect of RV and allergic sensitisation
RV infection may also act synergistically with allergic inflammation. For example, Calhoun et al9 demonstrated that patients with allergic rhinitis who were infected with experimental RV‐16 had enhanced histamine release into bronchoalveolar lavage (BAL) fluid immediately following antigen challenge, and this response was followed by increased eosinophil recruitment 48 hours later. The increase in airway eosinophils in response to antigen challenge persisted for up to 1 month after the infection. In addition, Grünberg et al15 found that RV infected patients had increased airway responsiveness to an inhaled histamine challenge. Conversely, when atopic patients were challenged with nasal allergens before inoculation of RV, the onset of symptoms was delayed with less severe responses.15 In addition, Murray et al16 found that the combination of virus detection and sensitisation with high allergen exposure in children substantially increased the risk of hospitalisation compared with virus detection or sensitisation and allergen exposure alone (OR 19.4, 95% CI 3.7 to 101.5; p<0.001). In a case‐control study of 60 adult hospitalised patients it was found that being sensitised and exposed to allergens was an independent risk factor for being admitted to hospital (OR 2.3).17 However, the combination of sensitisation, high exposure, and viral detection considerably increased the risk of being admitted with asthma (OR 8.4, 95% CI 2.1 to 32.8).17 It therefore appears that RV and allergen specific responses act together. This relationship may be stronger when RV infection occurs before allergen challenge.
Respiratory syncytial virus (RSV)
Traditionally, RSV has been considered the most frequent initiator of wheezing in children younger than 2 years of age and the major cause of bronchiolitis in infants.18 In children of this age, underlying allergy is not an apparent risk factor for wheezing with RSV. However, newer data with more sensitive molecular diagnostic techniques have indicated the contribution of early RV infections in the future development of subsequent wheezing. The role of RV may be particularly relevant in less severe infections as seen in an outpatient setting. For example, in a study of a high risk birth cohort of 289 infants, Lemanske et al19 found that first year wheezing illnesses caused by RV infections, but not RSV infection, were the strongest predictors of subsequent third year of life wheezing. Other studies have found similar results. RV infection has been shown to be as prevalent in causing bronchiolitis as RSV,20,21 and may cause a more severe course.22
Microbiology and immunopathogenesis
RSV is in the genus Pneumovirus in the family Paramyxoviridae. It is an enveloped medium sized virus with a genome consisting of single stranded RNA that encodes at least 10 different proteins.23 RSV has two major immunogens responsible for the formation of antibody: the fusion (F) protein and the attachment (G) protein. The G protein mediates the attachment of the virus to the epithelial cell and the F protein promotes the fusion of membranes for RNA insertion. Both murine and human studies have found that the reaction via the F protein gives rise to a predominantly Th1 immune response whereas the G protein appears to stimulate a Th2 immune response. Animal models have indicated that RSV has the potential to induce polarised cytokine response patterns (Th1 v Th2) on the basis of different reactions to these proteins.24 Based upon this polarising effect toward Th2 mediated airway inflammation, RSV is believed to worsen asthma symptoms in some children or, under some circumstances, contribute to the induction of allergy and asthma.
RSV has a predilection to infect, destroy, and biologically alter the lower airway epithelium. These properties may allow RSV to enhance allergic sensitisation with a consequential development of inflammation and responsiveness, as well as increase the direct actions of viral induced airway inflammation and hyperresponsiveness. In a murine model of RSV, Leibovitz et al25 determined that acute RSV infection correlated with increased concentrations of allergen specific IgE and IgG in serum and BAL fluid. Also, in a study of infants hospitalised with RSV, the investigators found suppression of IFN‐γ production which may facilitate a shift to Th2 activity and IgE sensitisation and eventual allergic inflammation.26
Influenza virus
Influenza can also precipitate an asthma exacerbation which can be quite severe. In one study, patients with influenza A induced asthma exacerbations had a decrease in forced expiratory volume in 1 second (FEV1) of more than 50%.27 Furthermore, the resulting decrease in FEV1 can persist for up to 90 days after infection.27 Given the cytopathic effects of influenza on airway tissue, it is not difficult to imagine how influenza may injure the lower airway, cause inflammation and, hence, worsen asthma.
Microbiology
Influenza viruses, members of the Orthomyxoviridae family, have rod shaped haemagglutinin and neuraminidase on their surface. These glycoproteins facilitate attachment to host cell and virus release, respectively.28 Necrosis of epithelial cells, oedema, and inflammation occur rapidly with influenza, and infection can spread into the smaller airways. Complete restoration of ciliary function and mucus production takes a minimum of 2 weeks.29
Immunopathogenesis
On a molecular level, influenza A infection induces large amounts of intrapulmonary IFN‐γ and enhances both later allergen specific asthma and dual Th1/Th2 responses.30 After intranasal inoculation with influenza A, mice mount a transient but robust Th1 cytokine response with subsequent pneumonia. Post‐influenza illness, mice which were sensitised to allergen had significantly increased numbers of pulmonary eosinophils, lymphocytes, and phagocytes. Airway hyperactivity also increased after the infection. Both IgE and IgG increased and IFN‐γ was shown to enhance both Th1 and Th2 mediated inflammation. In a study of children, Teran et al31 found that the eosinophil product major basic protein (MBP) and the chemoattractant RANTES increased with viral infection; the concentrations of RANTES correlated with clinical symptoms. Also, when respiratory epithelial cells were infected with influenza A, there was an increase in eotaxin mRNA expression and protein. Thus, eotaxin, and presumably eosinophil recruitment may be involved in the pathogenesis of airway inflammation in asthma exacerbations induced by influenza infection.32 After resolution of the acute infection, the recruited eosinophils may undergo degranulation to inflame the airways and prolong the effects of this infection on asthma, thus intensifying the underlying severity of the disease.
Human metapneumovirus
Human metapneumovirus (HMPV) has only recently been characterised but has a similar spectrum of disease to RSV, with clinical features of cough, coryza, fever, rhinitis, and wheezing. HMPV has proved to be a significant respiratory pathogen, accounting for many previously undiagnosed lower respiratory tract infections.33 For example, nasal wash specimens were collected from a population of 2009 infants and children who presented with a lower respiratory tract illness; 20% of those who previously tested “virus negative” (12% of all those with lower tract illness) contained HMPV. In addition, HMPV was found in 15% of upper respiratory tract infections that were previously of unknown aetiology. Thus, HMPV not only plays a significant role in respiratory infection but may be associated with worsening of asthma symptoms. The full spectrum of the effects of HMPV on asthma has yet to fully emerge.
Microbiology
The metapneumovirus genus is a member of the Pneumovirinae subfamily which is a member of the Paramyxoviridae family. Among common respiratory pathogens, HMPV is most closely related to RSV (a true pneumovirus). Metapneumoviruses differ from true pneumoviruses by having a slightly altered gene order and two fewer proteins.34
Immunopathogenesis
Since HMPV has only recently been described, its exact contribution to asthma exacerbations and its effect on asthma in general have yet to be fully investigated. Early work suggests a significant role for HMV in asthma, but its effect on the mechanisms of asthma has yet to be defined.
Bacterial infections
Mycoplasma pneumoniae
Mycoplasma pneumoniae is also a cause of acute respiratory infections in both children and adults and may exacerbate underlying asthma. Perhaps more importantly, some patients with asthma may develop a chronic infection with M pneumoniae which, in turn, could contribute to the persistence and severity of asthma.
Biscardi et al35 found that 20% of patients hospitalised for severe asthma also had a documented Mycoplasma infection. In 119 children aged 2–15 years admitted to hospital with asthma, M pneumonia infection was found in 20% of the subjects. Nasopharyngeal suction was obtained at admission and blood samples were obtained at admission and 2–4 weeks later. Diagnosis of M pneumoniae infection was based on positive serological testing consisting of specific IgM present in either blood sample or an increase of ⩾4‐fold in specific IgG. Of 51 patients experiencing their first asthma attack, over half had proven M pneumoniae infection. First asthma attacks that are coincident with a Mycoplasma infection largely occur among children with a family history of asthma or in those with high IgE levels. In this study, Mycoplasma infections that had not been treated with antibiotics were associated with recurrent exacerbations.
Similar data have been obtained with adults. Of 100 adults admitted to hospital with asthma, 18 had evidence of acute M pneumoniae infection compared with 3% of controls (p = 0.0006).36 Interestingly, all patients in this study had no evidence of pneumonia on chest radiography.
A recent review of this issue was undertaken by Johnston and Martin.37 Of the 12 studies examined that investigated atypical bacteria (M pneumoniae and/or C pneumoniae) in acute asthma, nine supported a relationship between infection and acute asthma. With a high proportion of studies linking infection with these pathogens and acute asthma, the authors concluded that these pathogens may play a significant role in such exacerbations.
Microbiology
Mycoplama pneumoniae is a member of the class Mollicutes and is the smallest free living prokaryote known to infect humans. It acts locally to destroy epithelial tissue, and tissue adherence occurs by its P1 attachment protein. Cytotoxic products such as hydrogen peroxide and superoxide anion are produced following infection, with respiratory cilia paralysis resulting from the illness. In some settings, prolonged (weeks to months) shedding of M pneumoniae from the respiratory tract may occur without any respiratory symptoms.
Immunopathogenesis
While the clinical significance of M pneumoniae in asthma is under investigation, the mechanisms leading to asthma exacerbations are not well defined. In a murine model, acute Mycoplasma infection increased airway resistance with increases in both Th1 and Th2 cytokines.38 Tumour necrosis factor (TNF)‐α, IFN‐γ, IL‐6, and IL‐8 were all significantly increased in infected mice with airway features typical of asthma. In mice sensitised to ovalbumin, infection with Mycoplasma elicited a Th2 response; in animals not sensitised to ovalbumin, a higher induction of IFN‐γ occurred.39 Therefore, in patients who have allergic sensitisation and asthma, infection with Mycoplasma may serve to enhance existing allergic inflammation and thus provoke an exacerbation of the underlying chronic asthma.
Chlamydia pneumoniae
Chlamydia pneumoniae impairs mucociliary clearance and increases mucus production in the lung. It is speculated that chronic C pneumoniae infection causes lower airway inflammation. In school aged children40 as well as adults,41C pneumoniae has been linked to asthma exacerbations and may be a contributor to the chronicity of the underlying disease. The finding that chronic chlamydial infections were more common in children with higher rates of asthma exacerbations suggests that a persistent Chlamydia infection increases susceptibility to other exacerbating stimuli (including allergens). It has also been proposed that the development of organism specific IgE antibody may cause mediator release leading to bronchospasm, airway inflammation, and bronchial hyperresponsiveness.42 More research is needed to elucidate the relationship between C pneumoniae infection, M pneumoniae infection, and asthma.
Allergens
Environmental allergens are important factors in many aspects of asthma. More than 80% of children with asthma are sensitised to environmental allergens.1 The ubiquity of allergens, together with the high rate of sensitisation to allergens in asthma, suggests that allergens play a significant role in asthma exacerbation.
Allergens represent a highly diverse collection of molecules that evoke an acute allergic response in sensitised individuals and, as a consequence of this response, cause respiratory symptoms to occur. It is also appreciated that prolonged exposure to aeroallergens can result in chronic airway inflammation via Th2 driven IgE mechanisms.43 Such an immunological reaction may intensify airway inflammation, increase activation of inflammatory cells, and stimulate mucus glands to hypersecrete—all of which can cause airway obstruction.
Studies of BAL fluid before and after allergen challenge show eosinophilic inflammation as the major airway response, associated with the late phase responses of airflow obstruction.44 In addition, IL‐5 and IL‐13 are significantly raised following allergen challenge. Interestingly, patients with concomitant increases in both Th1 and Th2 cytokines had greater eosinophilic airway inflammation. These cytokines were present 48 hours after antigen challenge, suggesting a possible mechanism for the prolonged allergic inflammation after antigen challenge. Thus, eosinophils are associated with allergen induced asthma exacerbations, and increases in both Th1 and Th2 cytokines are involved in the resolution of this response. Although the importance of eosinophils to the pathogenesis of asthma is no longer fully defined, sputum eosinophils are a major feature of asthma instability.45
There are many important aeroallergens that are classified as indoor, outdoor, and occupational. Common outdoor allergens include tree, weed and grass pollens, as well as fungi. Indoor allergens include allergens from mites, dogs, cats, and cockroaches. Rodents are a prominent source of occupational allergens. When proteins from these allergens are inhaled, they trigger an immunological response of airway cells to elicit inflammation and furthering of existing airway abnormalities.
Role of fungal allergy
When considering the role of allergens in acute asthma, fungi should receive special attention. Fungal allergens are found both outdoors and indoors, and sensitivity to these allergens is a risk factor for the development, persistence, severity, and mortality associated with asthma. Furthermore, fungal proteins can act as adjuvants in a Th2 driven response.46
Most fungal species are clinically relevant primarily as outdoor allergens. Fungal spores and particles are widely distributed throughout the world and form the majority of suspended biological particles in the air. Fungal spore counts are found year‐round in the southern part of the United States while, in cooler climates, outdoor levels of fungi diminish after the ground freezes. Alternaria spp spores predominate in temperate regions and are most common in dry, grain growing areas of the United States. Dispersion of spores occurs during dry weather periods and is accompanied by higher wind velocity and lower relative humidity.43
Although there are numerous important fungi involved in allergic disease, allergy to Alternaria alternata appears to be particularly relevant to asthma, its severity and exacerbations.46 In the second National Health and Nutrition Examination Survey (NHANES II), patients sensitised to Alternaria were approximately five times more likely to have asthma.47 Furthermore, Downs et al48 found that children sensitised to Alternaria had increased airway hyperresponsiveness, wheeze, and bronchodilator use compared with other atopic children.
Fungi and asthma severity
Sensitivity to fungi has also been linked to asthma severity as correlations exist between high airborne concentrations of fungi and emergency visits for asthma exacerbations.49 Additionally, severe and fatal asthma attacks are associated with allergy to Alternaria. O'Hollaren and colleagues50 investigated 11 patients (aged 11–25 years) with initial respiratory arrest in asthma (including two deaths) and 99 matched controls. Of the 11 patients with respiratory arrest, 10 had positive skin tests to Alternaria (p<0.001). After adjustment for age, Alternaria skin test reactivity was found to be associated with an approximate 200‐fold increase in the risk of respiratory arrest.50 Similar results were found in a larger study of 1132 adults.51
The frequency of sensitisation to moulds parallels asthma severity, a relationship not found with sensitivity to dust mites, pollens, or cats. Thus, sensitivity to Alternaria may play a significant role in asthma exacerbations, particularly for those with more severe disease.
Immunopathogenesis
In contrast to infection based asthma exacerbations, allergen induced asthmatic reactions evoke an IL‐5 response with increased eosinophil recruitment and degranulation. In addition, fungal associated proteases may lead to the development of allergic airway inflammation along with IgE mediated responses. As with many fungal allergens, Alternaria contains an intrinsic protease activity that can act as an adjuvant to prolong Th2 responses.46 Fungal extracts have been shown to cause epithelial cell shrinkage and desquamation.
Proteolytic degradation of cellular adhesion structures may explain this damage to the epithelium. Damage to the epithelial membrane then allows for easier passage of the allergen into the mucosa. Also, proteases can activate epithelial cells to increase pro‐inflammatory cytokine expression—that is, IL‐6 and IL‐852—which then leads to development of inflammation and subsequent asthma exacerbation.
Other causes of asthma exacerbation
Other causes of asthma exacerbation exist, including pollutants (such as tobacco smoke), ozone, and particulate matter, as well as occupational causes such as highly reactive chemicals, cleaning agents, animals, and latex. Although clinically important, a detailed discussion of these triggers is beyond the scope of this article.
Pollutants
Tobacco smoking is one of the most frequent exposures to an inhaled substance with adverse effects on the respiratory tract.43 Smoking adds particles and gases to indoor air and is a major source of fine particles in the air.53 Environmental tobacco smoke has been implicated in more severe RSV bronchiolitis,54 development of persistent wheezing,55 as well as asthma severity.56 Previous meta‐analyses and systemic reviews have confirmed the effect of exposure to environmental tobacco smoke on several respiratory symptoms and outcomes.57 The mechanisms of these effects are poorly understood.
Other outdoor air pollutants—such as other particulate matter, ozone, nitrogen dioxide, sulfur dioxide and diesel exhaust—can increase airway inflammation and airway responsiveness.58 The exact mechanisms by which air pollutants cause health effects are not well understood but, as suggested by Bernstein,58 may include free radical and oxidative stress, covalent modification, or stimulation of the nocioreceptor and autonomic nervous system; other possible mechanisms include ciliary dyskinesis, epithelial damage, and increased pro‐inflammatory mediators.59
Airway pollutants, together with viral infection, may actually work together to cause asthma exacerbations. Chauhan et al59 studied a cohort of 114 asthmatic children between 8 and 11 years of age. Participants used a diary card to record the best of three morning and evening prebronchodilator peak expiratory flow (PEF) rates as well as subjective assessments of respiratory symptoms. In addition, children wore personal nitrogen dioxide monitors. It was found that the severity of lower respiratory tract symptoms was increased and PEF measurements dropped with rising exposure to nitrogen dioxide in the week before infection.
Thus, as with infection and allergen exposure, pollutant exposure can augment the symptoms of infection based exacerbations. When both pollutant or allergen and infectious triggers continue, the destruction by one cause will augment the immune reaction to the other cause. Each process can damage the epithelial layer and initiate an immune response. Once primed, the inflammatory process continues with the additional exposure.
Occupational asthma
Occupational asthma has been implicated in 9–15% of cases of adult asthma60 and is characterised by variable airflow limitation and/or airway hyperresponsiveness due to causes and conditions attributable to a particular occupational environment.60 Agents causing occupational asthma include gases, fumes, dust, chemicals, cleaning agents and animals, and include more than 300 substances.60 These agents are categorised as high or low molecular weight. High molecular weight agents consist of proteins of animal or vegetable origin that act through an IgE mediated mechanism.61 Low molecular weight compounds may be organic or inorganic and normally do not react through IgE. Occupational asthma is an important contributor to asthma exacerbations, but a thorough review is beyond the scope of this article.
Conclusion
There are a number of aetiologies for asthma exacerbations that lead to the final common pathway of airway obstruction, increased airway hyperresponsiveness, bronchial inflammation, and mucus plugging. Whether it is the neutrophil infiltrate with viral infections, eosinophil infiltration with increased IL‐5 in allergen provocation, or the combination of multiple mechanisms, the identification of these aetiologies and their mechanisms will guide diagnosis, prognosis, and more specialised treatment based on the underlying cause of the exacerbation itself.
Abbreviations
BAL - bronchoalveolar lavage
FEV1 - forced expiratory volume in 1 second
HMPV - human metapneumovirus
ICAM‐1 - intercellular adhesion molecule 1
IL - interleukin
IFN‐γ - interferon γ
PEF - peak expiratory flow
RSV - respiratory syncytial virus
RV - rhinovirus
TNF‐α - tumour necrosis factor α
Footnotes
Competing interests: none declared.
References
- 1.Busse W W, O'Bryne P M, Holgate S T. Asthma pathogenesis. In: Adkinson NF Jr, Yunginger JW, Busse WW, et al eds. Middleton's allergy principles and practice. St Louis: Mosby, 20031175–1207.
- 2.Grissell T V, Powell H, Shafren D R.et al Interleukin‐10 gene expression in acute virus‐induced asthma. Am J Respir Crit Care Med 2005172433–439. [DOI] [PubMed] [Google Scholar]
- 3.Johnston S L, Pattemore P K, Sanderson G.et al Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 19953101225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson K G, Kent J, Ireland D C. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993307982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston S L, Pattemore P K, Sanderson G.et al The relationship between upper respiratory infections and hospital admissions for asthma: a time‐trend analysis. Am J Respir Crit Care Med 1996154654–660. [DOI] [PubMed] [Google Scholar]
- 6.Friedlander S L, Busse W W. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol 2005116267–273. [DOI] [PubMed] [Google Scholar]
- 7.Mosser A G, Brockman‐Schneider R, Amineva S.et al Similar frequency of rhinovirus‐infectible cells in upper and lower airway epithelium. J Infect Dis 2002185734–743. [DOI] [PubMed] [Google Scholar]
- 8.Gern J E. Rhinovirus respiratory infections and asthma. Am J Med 2002112(Suppl 6A)19–27S. [DOI] [PubMed] [Google Scholar]
- 9.Calhoun W J, Dick E C, Schwartz L B.et al A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest 1994942200–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corne J M, Lau L, Scott S J.et al The relationship between atopic status and IL‐10 nasal lavage levels in the acute and persistent inflammatory response to upper respiratory tract infection. Am J Respir Crit Care Med 20011631101–1107. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos N G, Stanciu L A, Papi A.et al A defective type 1 response to rhinovirus in atopic asthma. Thorax 200257328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wark P A, Johnston S L, Bucchieri F.et al Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005201937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parry D E, Busse W W, Sukow K A.et al Rhinovirus‐induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol 2000105692–698. [DOI] [PubMed] [Google Scholar]
- 14.Gern J E, Vrtis R, Grindle K A.et al Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med 20001622226–2231. [DOI] [PubMed] [Google Scholar]
- 15.Grunberg K, Timmers M C, Smits H H.et al Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin‐8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy 19972736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray C S, Poletti G, Kebadze T.et al Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospitalization in children. Thorax 200661376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green R M, Custovic A, Sanderson G.et al Synergism between allergens and viruses and risk of hospital admission with asthma: case‐control study. BMJ 2002324763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemanske R F., Jr Viruses and asthma: inception, exacerbation, and possible prevention. J Pediatr 2003142S3–S7. [DOI] [PubMed] [Google Scholar]
- 19.Lemanske R F, Jr, Jackson D J, Gangnon R E.et al Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005116571–577. [DOI] [PubMed] [Google Scholar]
- 20.Korppi M, Kotaniemi‐Syrjanen A, Waris M.et al Rhinovirus‐associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J 200423995–999. [DOI] [PubMed] [Google Scholar]
- 21.Jartti T, Lehtinen P, Vuorinen T.et al Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis 2004101095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulos N G, Moustaki M, Tsolia M.et al Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med 20021651285–1289. [DOI] [PubMed] [Google Scholar]
- 23.Tristram D A, Welliver R C. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and practice of pediatric infectious diseases. Philadelphia: Churchill Livingstone, 20031140–1148.
- 24.Schwarze J, Gelfand E W. Respiratory viral infections as promoters of allergic sensitization and asthma in animal models. Eur Respir J 200219341–349. [DOI] [PubMed] [Google Scholar]
- 25.Leibovitz E, Freihorst J, Piedra P A.et al Modulation of systemic and mucosal immune responses to inhaled ragweed antigen in experimentally induced infection with respiratory syncytial virus implication in virally induced allergy. Int Arch Allergy Appl Immunol 198886112–116. [DOI] [PubMed] [Google Scholar]
- 26.Aoyagi M, Shimojo N, Sekine K.et al Respiratory syncytial virus infection suppresses IFN‐gamma production of gammadelta T cells. Clin Exp Immunol 2003131312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan R A, Geddes D, Woodhead M. Management of influenza in patients with asthma or chronic obstructive pulmonary disease. Ann Allergy Asthma Immunol. 2001;87: 447–54, 487, [DOI] [PubMed]
- 28.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med 20053531363–1373. [DOI] [PubMed] [Google Scholar]
- 29.Subbarao K. Influenza viruses. In: Long SS, Pickering LK, Prober CG, eds. Principles and practice of pediatric infectious diseases. Philadelphia: Churchill Livingstone, 20031159–1166.
- 30.Dahl M E, Dabbagh K, Liggitt D.et al Viral‐induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol 20045337–343. [DOI] [PubMed] [Google Scholar]
- 31.Teran L M, Seminario M C, Shute J K.et al RANTES, macrophage‐inhibitory protein 1α, and the eosinophil product major basic protein are released into upper respiratory secretions during virus‐induced asthma exacerbations in children. J Infect Dis 1999179677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi M, Kokubu F, Kuga H.et al Expression of eotaxin by normal airway epithelial cells after influenza virus A infection. Int Arch Allergy Immunol 2000122(Suppl 1)44–49. [DOI] [PubMed] [Google Scholar]
- 33.Williams J V, Harris P A, Tollefson S J.et al Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004350443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh K, McAdam A J. Human metapneumovirus—an important new respiratory virus. N Engl J Med 2004350431–433. [DOI] [PubMed] [Google Scholar]
- 35.Biscardi S, Lorrot M, Marc E.et al Mycoplasma pneumoniae and asthma in children. Clin Infect Dis 2004381341–1346. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman D, Lieberman D, Printz S.et al Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med 2003167406–410. [DOI] [PubMed] [Google Scholar]
- 37.Johnston S L, Martin R J. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med 20051721078–1089. [DOI] [PubMed] [Google Scholar]
- 38.Hardy R D, Jafri H S, Olsen K.et al Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect Immun 2001693869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu H W, Honour J M, Rawlinson C A.et al Effects of respiratory Mycoplasma pneumoniae infection on allergen‐induced bronchial hyperresponsiveness and lung inflammation in mice. Infect Immun 2003711520–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham A F, Johnston S L, Julious S A.et al Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J 199811345–349. [DOI] [PubMed] [Google Scholar]
- 41.Miyashita N, Kubota Y, Nakajima M.et al Chlamydia pneumoniae and exacerbations of asthma in adults. Ann Allergy Asthma Immunol 199880405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emre U, Sokolovskaya N, Roblin P M.et al Detection of anti‐Chlamydia pneumoniae IgE in children with reactive airway disease. J Infect Dis 1995172265–267. [DOI] [PubMed] [Google Scholar]
- 43.Peden D B. Air pollution: indoor and outdoor. In: Adkinson NF Jr, Yunginger JW, Busse WW, et al eds. Middleton's allergy principles and practice. St Louis: Mosby, 2003515–528.
- 44.Liu L, Jarjour N N, Busse W W.et al Enhanced generation of helper T type 1 and 2 chemokines in allergen‐induced asthma. Am J Respir Crit Care Med 20041691118–1124. [DOI] [PubMed] [Google Scholar]
- 45.Bartoli M L, Bacci E, Carnevali S.et al Clinical assessment of asthma severity partially corresponds to sputum eosinophilic airway inflammation. Respir Med 200498184–193. [DOI] [PubMed] [Google Scholar]
- 46.Bush R K, Prochnau J J. Alternaria‐induced asthma. J Allergy Clin Immunol 2004113227–234. [DOI] [PubMed] [Google Scholar]
- 47.Gergen P J, Turkeltaub P C. The association of individual allergen reactivity with respiratory disease in a national sample: data from the second National Health and Nutrition Examination Survey, 1976–80 (NHANES II). J Allergy Clin Immunol 199290579–588. [DOI] [PubMed] [Google Scholar]
- 48.Downs S H, Mitakakis T Z, Marks G B.et al Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med 2001164455–459. [DOI] [PubMed] [Google Scholar]
- 49.Rosas I, McCartney H A, Payne R W.et al Analysis of the relationships between environmental factors (aeroallergens, air pollution, and weather) and asthma emergency admissions to a hospital in Mexico City. Allergy 199853394–401. [DOI] [PubMed] [Google Scholar]
- 50.O'Hollaren M T, Yunginger J W, Offord K P.et al Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med 1991324359–363. [DOI] [PubMed] [Google Scholar]
- 51.Zureik M, Neukirch C, Leynaert B.et al Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community Respiratory Health Survey. BMJ 2002325411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kauffman H F, Tomee J F, van de Riet M A.et al Protease‐dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol 20001051185–1193. [DOI] [PubMed] [Google Scholar]
- 53.Gautrin D, Infante‐Rivard C, Ghezzo H.et al Incidence and host determinants of probable occupational asthma in apprentices exposed to laboratory animals. Am J Respir Crit Care Med 2001163899–904. [DOI] [PubMed] [Google Scholar]
- 54.Kurz T, Ober C. The role of environmental tobacco smoke in genetic susceptibility to asthma. Curr Opin Allergy Clin Immunol 20044335–339. [DOI] [PubMed] [Google Scholar]
- 55.Copenhaver C C, Gern J E, Li Z.et al Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med 2004170175–180. [DOI] [PubMed] [Google Scholar]
- 56.Eisner M D, Klein J, Hammond S K.et al Directly measured second hand smoke exposure and asthma health outcomes. Thorax 200560814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brims F, Chauhan A J. Air quality, tobacco smoke, urban crowding and day care: modern menaces and their effects on health. Pediatr Infect Dis J 200524S152–S156. [DOI] [PubMed] [Google Scholar]
- 58.Bernstein J A, Alexis N, Barnes C.et al Health effects of air pollution. J Allergy Clin Immunol 20041141116–1123. [DOI] [PubMed] [Google Scholar]
- 59.Chauhan A J, Inskip H M, Linaker C H.et al Personal exposure to nitrogen dioxide (NO2) and the severity of virus‐induced asthma in children. Lancet 20033611939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mapp C E, Boschetto P, Maestrelli P.et al Occupational asthma. Am J Respir Crit Care Med 2005172280–305. [DOI] [PubMed] [Google Scholar]
- 61.Chan‐Yeung M, Malo J L. Aetiological agents in occupational asthma. Eur Respir J 19947346–371. [DOI] [PubMed] [Google Scholar]