Abstract
Background
Skeletal muscle wasting commonly occurs in patients with chronic obstructive pulmonary disease (COPD) and has been associated with the presence of systemic inflammation. This study investigated whether rehabilitative exercise training decreases the levels of systemic or local muscle inflammation or reverses the abnormalities associated with muscle deconditioning.
Methods
Fifteen patients with COPD (mean (SE) forced expiratory volume in 1 s 36 (4)% predicted) undertook high‐intensity exercise training 3 days/week for 10 weeks. Before and after the training programme the concentration of tumour necrosis factor α (TNFα), interleukin‐6 (IL‐6) and C‐reactive protein (CRP) in plasma was determined by ELISA, and vastus lateralis mRNA expression of TNFα, IL‐6, total insulin‐like growth factor‐I (IGF‐I) and its isoform mechanogrowth factor (MGF) and myogenic differentiation factor D (MyoD) were assessed by real‐time PCR. Protein levels of TNFα, IGF‐I and MyoD were measured by Western blotting.
Results
Rehabilitation improved peak exercise work rate by 10 (2%) (p = 0.004) and mean fibre cross‐sectional area from 4061 (254) μm2 to 4581 (241) μm2 (p = 0.001). Plasma inflammatory mediators and vastus lateralis expression of TNFα and IL‐6 were not significantly modified by training. In contrast, there was a significant increase in mRNA expression of IGF‐I (by 67 (22)%; p = 0.044), MGF (by 67 (15)%; p = 0.002) and MyoD (by 116 (30)%; p = 0.001). The increase observed at the mRNA level was also seen at the protein level for IGF‐I (by 72 (36)%; p = 0.046) and MyoD (by 67 (21)%; p = 0.012).
Conclusions
Pulmonary rehabilitation can induce peripheral muscle adaptations and modifications in factors regulating skeletal muscle hypertrophy and regeneration without decreasing the levels of systemic or local muscle inflammation.
Inflammatory activation with increased serum levels of mediators such as tumour necrosis factor α (TNFα), interleukin (IL)‐6 and acute phase reactant proteins such as C‐reactive protein (CRP) have been described1,2 as important factors in the progression of chronic obstructive pulmonary disease (COPD). Although the involvement of systemic cytokines (such as TNFα and IL‐6) in peripheral muscle dysfunction has never been directly documented in COPD, the available literature strongly supports a casual relationship.1,2,3,4 Importantly, in two studies5,6 enhanced levels of TNFα have been found in the skeletal muscle of patients with COPD compared with age‐matched healthy controls, whereas in another study local muscle TNFα could not be detected.7
Recent experimental data suggest that TNFα interferes with the action of muscle insulin‐like growth factor‐I (IGF‐I), thereby leading to muscle atrophy.8 In addition, TNFα inhibits myogenic differentiation through destabilising myogenic differentiation factor D (MyoD) protein, thus interfering with skeletal muscle regeneration.9 Furthermore, in a rodent local infusion model, IL‐6 resulted in muscle atrophy characterised by preferential loss of myofibrillar protein.10
Skeletal muscle dysfunction is common in patients with advanced COPD and it contributes importantly to limiting their functional capacity and quality of life.11 Morphological and biochemical changes within the vastus lateralis muscle of these patients include abnormal fibre‐type distribution, reduced fibre cross‐sectional areas and decreased muscle capillarity and oxidative enzyme activities.12 We recently reported that both constant‐load and interval training modalities improve exercise capacity by reversing, at least in part, the aforementioned muscle abnormalities.13 It remains, however, unknown whether exercise training simply reverses the abnormalities associated with muscle deconditioning or actually interferes with certain inflammatory factors, such as TNFα and IL‐6, that have been proposed to be involved in the pathogenesis of COPD‐related muscle dysfunction.1,2,3,4
Rabinovich and colleagues5 were the first to report that exercise training does not decrease the levels of systemic or local muscle TNFα in patients with COPD; this report, however, did not provide direct evidence as to whether training actually slowed or even reversed the peripheral muscle abnormalities. On the other hand, although there are studies reporting no effect of exercise training on systemic levels of IL‐6,5,14 the impact of pulmonary rehabilitation on the expression of local muscle IL‐6 is unknown.
Interestingly, exercise training in healthy elderly individuals15,16,17,18 and in patients with chronic heart failure19 has been shown to be associated with upregulation of mRNA expression of total IGF‐I, its load‐sensitive splice variant termed mechanogrowth factor (MGF) and MyoD, all of which are important factors for skeletal muscle hypertrophy and regeneration. The possibility that this mechanism could also be an underlying cause of muscle adaptation in patients with COPD has not been investigated.
This study was therefore undertaken to investigate the effect of training on the local muscle expression of IGF‐I, MGF and MyoD. The effect of training on systemic (plasma TNFα, IL‐6 and CRP) and local muscle inflammation (expression of TNFα and IL‐6) was also investigated. It was hypothesised that the training‐induced skeletal muscle adaptations are accompanied by increased local muscle IGF‐I, MGF and MyoD expression, but not with reductions of systemic or local muscle inflammation. The latter was based on the results of previous studies in COPD showing a lack of change in systemic and/or local muscle inflammation with exercise training.5,14
Methods
Study population
Fifteen patients (three women) with clinically stable COPD met the following criteria: (1) post‐bronchodilator forced expiratory volume in 1 s (FEV1) <50% predicted and FEV1/forced vital capacity (FVC) <70% without significant post‐bronchodilator reversibility (<10% FEV1 % predicted normal); (2) optimal medical treatment according to GOLD20 without regular use of systemic corticosteroids; and (3) absence of other significant diseases that could contribute to exercise limitation. Four of these patients had also participated in a previous report.13 Ten age‐matched subjects with FEV1 >92% predicted were also included in the present study. Patients and healthy subjects gave written informed consent that was approved by the university ethics committee.
Study design
Similar to our previous study,13 patients were admitted to an ongoing randomised controlled pulmonary rehabilitation trial consisting of two types of intensive exercise training (interval and constant‐load) lasting 10 consecutive weeks. Since our previous work established no differences between training modalities in terms of muscle morphological characteristics,13 the present study aimed to determine the effect of exercise training per se on local muscle inflammatory and myogenic factors. Prior to and on completion of the programme, patients were assessed for pulmonary function, exercise tolerance and plasma inflammatory mediator levels. A muscle percutaneous biopsy was also performed.
Assessments
Assessments included: (1) resting pulmonary function, carbon monoxide transfer factor (Tlco) and subdivisions of lung volumes by body plethysmography (Medgraphics Autolink 1085D, Minnesota, USA); (2) arterial blood analysis (ABL330; Radiometer, Copenhagen, Denmark); (3) incremental (increments of 5–10 W/min) cycle ergometer exercise (Ergoline 800; Sensor Medics, California, USA) to the limit of tolerance (Wpeak). During the test the flow rate at the mouth and gas exchange variables were recorded breath‐by‐breath (Vmax 229; Sensor Medics). Cardiac frequency and percentage oxygen saturation were determined using the R–R interval from a 12‐lead online ECG (Marquette Max; Marquette Hellige GmbH, Germany) and a pulse oximeter (Nonin 8600; Nonin Medical, USA), respectively. Symptom ratings were monitored every 2 min throughout exercise using the 1–10 Borg scale.21
Exercise training programme
Patients performed high‐intensity exercise either at a constant load (initially set at 60% Wpeak for 30 min; n = 8) or at intervals of 30 s work alternating with 30 s rest (load initially set at 100% Wpeak, for 45 min, n = 7). Exercise was performed on electromagnetically braked ergometers (CatEye‐Ergociser, EC‐1600; Osaka, Japan) as previously described.13,22,23 The workload was increased on a weekly basis as detailed below. As in our previous rehabilitation studies,13,23 the exercise prescription was designed to present patients with a similar overall training load.
Plasma inflammatory mediators
Plasma was collected using EDTA 24 h before and 24 h after the rehabilitation programme. The concentration of mediators was determined by ELISA according to the manufacturer's instructions (TNFα, IL‐6 and CRP; R&D Systems, Minneapolis, Minnesota, USA). Samples of healthy controls were assayed together with the patient samples. The sensitivity of the respective assays was 0.12 pg/ml for TNFα, 0.04 pg/ml for IL‐6 and 0.01 ng/ml for CRP.
Muscle biopsy
Previous work24 has shown that the mRNA content of both IGF‐I and MyoD 24 h after an exercise bout does not differ from that before the exercise bout. Muscle percutaneous biopsy specimens of the right vastus lateralis muscle performed at mid‐thigh (15 cm above the patella) were therefore obtained 24 h before the first and 24 h after the last training session and analysed blindly as previously described13 for fibre type classification and cross‐sectional areas. In brief, muscle samples were cut into two pieces. One was placed immediately into liquid nitrogen and the other was aligned under a stereoscope in order to have most of the fibres in parallel. It was then placed in embedding compound and frozen in isopentane precooled to its freezing point. All biopsy specimens were kept at −80°C until the day of analysis. Cryostat transverse sections 10 μm thick from the embedded samples were cut at −20°C and stained for myofibrillar ATPase after pre‐incubation at pH 4.3, 4.6 and 10.3.25 A mean (SE) of 413 (39) muscle fibres were classified as type I, IIa or IIb from each sample. The cross‐sectional area of at least 300 fibres from each sample was measured with an image analysis system (ImagePro; Media Cybernetics Inc, Silver Spring, Maryland, USA) at a known and calibrated magnification. The mean cross‐sectional area of all fibre types was calculated, taking into account the relative contribution of each fibre type to the total surface.7
Quantitative real‐time PCR
Total RNA was extracted from 30 mg muscle biopsy specimens using an RNeasy Fibrous Tissue (Qiagen, West Sussex, UK). The total RNA was then quantified (260 nm) and adjusted to a concentration of 1 μg/μl. The cDNA was synthesised using 1 μg total RNA from each sample using a SuperScript First‐Strand Synthesis System (Invitrogen, Carlsbad, California, USA) for real‐time PCR according to the manufacturer's instructions. 2 μl of each cDNA sample were used as a template for the amplification reaction using SyBR greenER qPCR Supermix Universal. Primer sequences for TNFα, IL‐6, IGF‐I total (IGF‐IEabc), MGF (IGF‐IEc) and MyoD are given in table 1 (in the online supplement available at http://thorax.bmj.com/supplemental). PCR amplifications were performed in triplicate in a Chromo4 Detector and PTC‐200 Peltier Thermal Cycler and analysed with Opticon software 2.03 (MJ Research, Massachusetts, USA). The threshold cycle (CT value) was chosen as the first amplification cycle giving a signal above background. To calculate the relative quantity of the respective genes, the ΔΔCT method was used.26 Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA expression was used for normalisation.27 More information on real‐time PCR procedures is given in the online supplement (http://thorax.bmj.com/supplemental).
Table 1 Demographic and lung function characteristics of patients with COPD (n = 15) and age‐matched healthy subjects (n = 10).
| COPD | Controls | |
|---|---|---|
| Age (years) | 66 (7) | 61 (5) |
| BMI (kg/m2) | 25.9 (2.7) | 28.8 (2.6) |
| FEV1 (l) [% pred] | 0.94 (0.44) [35.7 (16.4)] | 2.86 (0.8) [94.7 (5.4)]* |
| FVC (l) [% pred] | 2.47 (0.82) [71.3 (20.0)] | 3.76 (0.9) [101.3 (11)]* |
| FEV1/FVC (%) | 36 (11) | 77 (4)* |
| Tlco (% pred) | 40.2 (16.8) | 81.2 (5.9)* |
| TLC (l) [% pred] | 7.92 (1.24) [126 (9)] | 6.16 (0.21) [95 (3)]* |
| FRC (l) [% pred] | 5.83 (0.76) [172 (14)] | 3.22 (0.11) [96 (4)]* |
| RV (l) [% pred] | 4.52 (0.82) [209 (30)] | 1.93 (0.09) [88 (7)]* |
| IC (l) [% pred] | 2.09 (0.60) [72 (10)] | 2.96 (0.18) [92 (5)]* |
| Pao2 (mmHg) | 65.1 (9.5) | _ |
| Paco2 (mmHg) | 39.8 (2.6) | _ |
| pH | 7.44 (0.20) | _ |
| Sao2 (%) | 92.8 (2.2) | _ |
Values are mean (SD).
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; Tlco, carbon monoxide transfer factor; TLC, total lung capacity; FRC, functional residual capacity; RV, residual volume; IC, inspiratory capacity; Pao2, Paco2, arterial oxygen and carbon dioxide tensions; Sao2, oxygen saturation.
*p<0.05 vs COPD (unpaired t test).
Muscle protein immunoblotting
Vastus lateralis muscle biopsies stored at −80°C were homogenised in 10 volumes (wt/vol) of a lysis buffer as previously described.26 The protein concentration was determined using a DC protein assay (BioRad, Hercules, California, USA). Samples were subjected to SDS polyacrylamide gel electrophoresis (SDS‐PAGE) followed by transfer to a polyvinylidene fluoride membrane (PVDF) (Millipore Corp, Bedford, Massachusetts, USA). Immunoblotting was carried out using primary antibodies raised against MyoD (1:200, Santa Cruz Biotechnology, Santa Cruz, California, USA), total IGF‐I (1:200, Santa Cruz Biotechnology) and TNFα (1:1000, Santa Cruz Biotechnology). To validate equal protein loading among the various lanes, PVDF membranes were stripped and re‐probed with a monoclonal anti‐sarcomeric α‐actinin antibody (1:1000, Sigma‐Aldrich, St Louis, Missouri, USA).28 All bands were visualised using Chemiluminescent Substrate (Pierce, Rockford, Illinois, USA). Data were digitalised and quantified by densitometric analysis. More information on protein immunoblotting is given in the online supplement available at http://thorax.bmj.com/supplemental.
Statistical analysis
The minimum sample size was calculated based on 80% power and a two‐sided 0.05 significance level using the Statistica 7.0 statistical program. Sample size capable of detecting a change of 3 pg/ml for TNFα and IL‐6 plasma levels and of 30% for the TNFα mRNA expression was estimated using data obtained from a previous study5 and the following standard deviations: TNFα, 9 pg/ml; IL‐6, 7 pg/ml; TNFα mRNA, 25 TNFα/18S.The critical sample size was estimated to be 14 patients. Data are presented as mean (SD) for the subject characteristics and as mean (SE) for all other variables. Mean differences between pre‐ and post‐rehabilitation (with 95% confidence intervals (CI)) are given for the plasma inflammatory mediators and local muscle mRNA and protein expression. Comparisons of baseline plasma inflammatory levels, demographic and lung function characteristics between patients with COPD and healthy subjects were performed using an unpaired t test. Pre‐ and post‐training comparisons were performed using the Student paired t test (exercise testing data, muscle morphological characteristics, plasma and local muscle TNFα and IL‐6) and the Wilcoxon signed rank test (plasma CRP and local muscle mRNA expression of IGF‐I, MGF and MyoD), depending on whether or not data were normally distributed. A one‐sample t test was used to identify whether the differences in protein levels before and after rehabilitation were significant. The level of significance was set at p<0.05.
Results
Baseline patient characteristics
Patients were characterised by severe airflow limitation, hypoxaemia without carbon dioxide retention at rest, reduced transfer factor and lung hyperinflation, as reflected by increased functional residual capacity (table 1). Age and BMI were not significantly different between patients with COPD and healthy controls (table 1).
Exercise training programme
Examination of the mean training intensity (interval = 115 (15)% and constant‐load = 70 (5)% of baseline Wpeak) revealed that the total amount of work sustained during interval and constant‐load training was comparable. Mean training intensity increased progressively throughout the rehabilitation programme such that, at weeks 3, 6 and 10, it corresponded to 100 (13)%, 115 (13)% and 130 (16)% of baseline Wpeak for interval training and to 62 (3)%, 70 (5)% and 81 (5)% of baseline Wpeak for constant‐load training.
Exercise capacity
After rehabilitation there was a significant improvement in peak values of external work rate, oxygen uptake, lactate threshold and minute ventilation (table 2).
Table 2 Peak exercise responses before and after training in patients with COPD (n = 15).
| Before training | After training | |
|---|---|---|
| Work rate (W) | 50 (6) | 55 (6)* |
| V̇o2 (ml/kg/min) | 12.1 (1.1) | 13.3 (1.4)* |
| LT (ml/kg/min) | 8.5 (0.6) | 10.7 (2.4)* |
| V̇e (l/min) | 35.9 (3.2) | 37.1 (3.1)* |
| fb (breaths/min) | 31 (2) | 34 (2)* |
| Vt (l) | 1.15 (0.08) | 1.16 (0.09) |
| Spo2 (%) | 93 (1) | 90 (1) |
| RER | 0.98 (0.04) | 0.99 (0.04) |
| Dyspnoea (Borg) | 3.8 (0.4) | 4.4 (0.4) |
| Leg fatigue (Borg) | 3.9 (0.4) | 3.6 (0.5) |
Values are mean (SE).
WR, work rate; V̇o2, oxygen uptake; LT, lactate threshold; V̇e, minute ventilation; fb, breathing frequency; Vt, tidal volume; Spo2%, percentage arterial oxygen saturation; RER, respiratory exchange ratio.
*p<0.05 vs before training (paired t test).
Skeletal muscle adaptations
The proportion of type I and type IIa fibres did not change significantly after training, whereas the proportion of type IIb fibres was significantly reduced (p = 0.001, table 3). Following rehabilitation the cross‐sectional area of each fibre type was significantly increased (table 3), so the mean fibre cross‐sectional area also increased significantly (from 4061 (254) μm2 to 4581 (241) μm2; p = 0.001).
Table 3 Effect of training on vastus lateralis characteristics in patients with COPD (n = 15).
| Before training | After training | |
|---|---|---|
| Fibre type distribution (%) | ||
| Type I | 37 (4) | 39 (4) |
| Type IIa | 49 (4) | 53 (5) |
| Type IIb | 14 (3) | 8 (1)* |
| Cross‐sectional area (μm2) | ||
| Type I | 4606 (332) | 5170 (372)* |
| Type IIa | 4225 (295) | 4711 (264)* |
| Type IIb | 3236 (199) | 3693 (209)* |
Values are mean (SE).
*p<0.05 vs before training (paired t test).
Plasma TNFα, IL‐6 and CRP
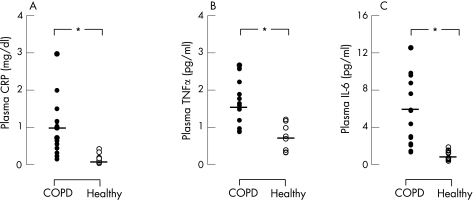
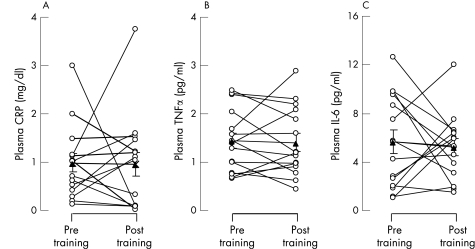
In patients with COPD the plasma concentrations of TNFα (1.44 (0.17) pg/ml), IL‐6 (5.68 (0.97) pg/ml) and CRP (0.97 (0.19) mg/dl) before training were significantly (p = 0.001) greater (by 3, 8 and 7‐fold, respectively) than in healthy subjects (0.53 (0.12) pg/ml, 0.76 (0.17) pg/ml and 0.15 (0.04) mg/dl, respectively; fig 1). Following rehabilitation, the mean difference in TNFα (−0.10 (95% CI −0.34 to 0.13) pg/ml), IL‐6 (−0.59 (95% CI −2.8 to 1.7) pg/ml) and CRP (−0.15 (95% CI −0.7 to 0.6) mg/dl) in patients with COPD was not significant (fig 2).
Figure 1 Individual (circles) and mean group (lines) values of plasma levels of (A) C‐reactive protein (CRP), (B) tumour necrosis factor‐α (TNFα) and (C) interleukin‐6 (IL‐6) in patients with chronic obstructive pulmonary disease (COPD, n = 15; closed circles) and healthy age‐matched subjects (n = 10; open circles).
Figure 2 Individual (circles) and mean (SE) group (triangles) values of plasma levels of (A) C‐reactive protein (CRP), (B) tumour necrosis factor‐α (TNFα) and (C) interleukin‐6 (IL‐6) before and after training in patients with chronic obstructive pulmonary disease (COPD, n = 15).
Skeletal muscle mRNA expression
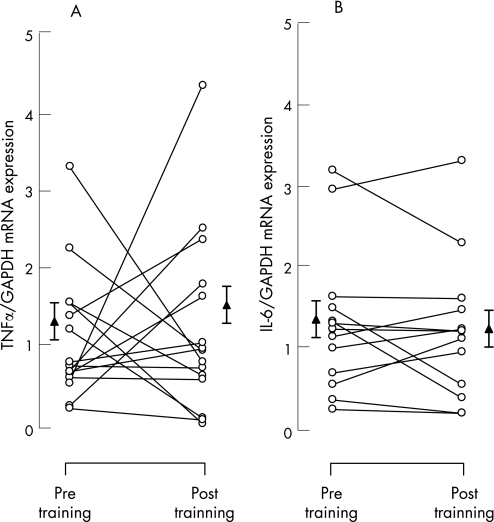
The mRNA contents were normalised for GAPDH24,27 and the results are given in relative units. Following exercise training, local muscle TNFα mRNA expression was not significantly different from the level before training (1.24 (0.19) vs 1.37 (0.26), p = 0.73, fig 3A), corresponding to a mean difference from baseline of 10 (22)% (95% CI −36% to 57%). IL‐6 mRNA expression was also not significantly changed after training (1.38 (0.22) vs 1.29 (0.22), p = 0.50, fig 3B). The mean difference from baseline was −2 (12)% (95% CI −29% to 25%).
Figure 3 Individual (circles) and mean (SE) group (triangles) effects of training on vastus lateralis mRNA expression of (A) tumour necrosis factor‐α (TNFα) and (B) interleukin‐6 (IL‐6) (n = 15). Data are normalised for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA. *p<0.05 compared with pre‐training values.
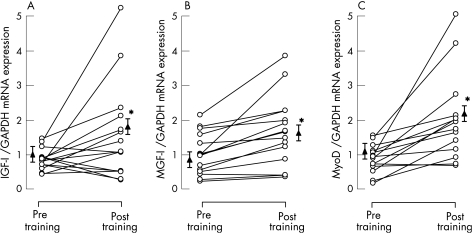
Vastus lateralis total IGF‐I mRNA expression was significantly increased after training (from 1.02 (0.07) to 1.77 (0.40), p = 0.044, fig 4A), corresponding to an increase of 67 (22)% (95% CI 3% to 117%). MGF mRNA expression was also significantly increased after training (from 0.92 (0.16) to 1.50 (0.27), p = 0.002, fig 4B), corresponding to 67 (15)% (95% CI 34% to 100%) and, similarly, local muscle MyoD mRNA expression was significantly upregulated in response to training (from 1.18 (0.22) to 2.20 (0.33), p = 0.001, fig 4C) or by 116 (30)% (95% CI 51% to 181%).
Figure 4 Individual (circles) and mean (SE) group (triangles) effects of training on vastus lateralis mRNA expression of (A) insulin‐like growth factor‐I (IGF‐I), (B) mechanogrowth factor (MGF) and (C) MyoD (n = 15). Data are normalised for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA. *p<0.05 compared with pre‐training values.
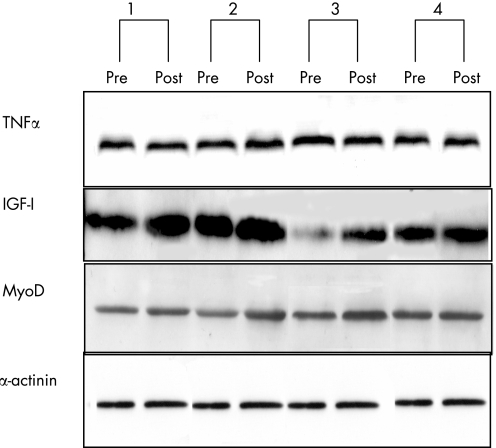
Skeletal muscle TNFα protein
To test whether the changes observed at the mRNA level were also extended to the protein level, muscle tissue was analysed by Western blotting. Representative Western blots are shown in fig 5. Based on densitometric analysis, it was determined that expression of TNFα was not significantly changed after rehabilitation (9 (24)% (95% CI −49% to 66%), p = 0.74). In contrast, after training there was a significant increase in the expression of both IGF‐I (by 72 (36)% (95% CI −12% to 155%), p = 0.046) and MyoD (by 67 (21)% (95% CI 18% to 116%), p = 0.012; fig 5).
Figure 5 Representative pre‐and post‐training Western blots for four patients for local muscle tumour necrosis factor α (TNFα), insulin‐like growth factor‐I (IGF‐I) and MyoD relative to α‐actinin antibody. Data quantified by densitometric analysis.
Discussion
Although the involvement of systemic inflammation in peripheral muscle dysfunction has never been directly documented in COPD, the available literature strongly supports a casual relationship.1,2,3,4 Regular exercise training is known to partially reverse the peripheral muscle abnormalities,12,13 but it is uncertain whether this is the result of reversing the effects of deconditioning alone or if muscle reconditioning actually decreases the peripheral muscle inflammation. The results of the present study show that lower limb exercise training induces significant adaptations in vastus lateralis fibre size and typology without reducing the systemic inflammatory mediator levels or the local muscle expression of TNFα and IL‐6. As these skeletal muscle adaptations were accompanied by significant increase in factors regulating skeletal muscle hypertrophy and regeneration (ie, local muscle total IGF‐I, MGF and MyoD expression), it is suggested that the peripheral muscles of patients with COPD retain adequate plasticity for remodelling in response to exercise training.
We investigated the effects of peripheral muscle training on specific systemic inflammatory mediator levels (TNFα, IL‐6 and CRP) which have been proposed to be associated with muscle dysfunction in COPD.1,2,3,4 Compared with healthy age‐matched controls, the patients exhibited significantly raised plasma levels of TNFα (3‐fold), IL‐6 (8‐fold) and CRP (7‐fold). The fold increase of these mediators before rehabilitation in our COPD patients relative to healthy controls are in line with those previously reported in patients with stable COPD of similar severity and body mass index.2,29,30 Although lack of change in systemic levels of TNFα and IL‐6 with exercise training has previously been reported,5,14 to our knowledge this is the first study in patients with COPD to report a lack of effect of rehabilitation on systemic CRP levels. This is an interesting finding since CRP has been implicated as a marker for impairment in exercise capacity and respiratory distress.2
With the present rehabilitation programme, local muscle TNFα mRNA and protein levels were not significantly changed, thus expanding the results of Rabinovich et al5 who found unchanged levels of muscle TNFα mRNA expression in patients on completion of a similar endurance training programme. In addition, the current study is the first to our knowledge to examine the effects of training on vastus lateralis muscle IL‐6 mRNA expression in patients with COPD. The lack of change in local muscle IL‐6 mRNA expression corroborates previous results in healthy individuals that exercise training does not effect local muscle Il‐6 mRNA expression.31
In the study by Rabinovich et al5 a lack of training‐induced downregulation of muscle TNFα mRNA expression was attributed to the lower muscle antioxidant capacity in COPD compared with healthy controls.32 Furthermore, Mercken et al33 have shown that patients with COPD have higher levels of systemic and pulmonary oxidative stress markers after endurance exercise than age‐matched healthy subjects and, although they tend to be reduced after pulmonary rehabilitation, they remain at higher levels than in healthy controls. Since muscle inflammation, tissue hypoxaemia and oxidative stress are strongly associated in COPD,3,4 it is possible that, with the present training programme, the repeated intense exercise sessions in chronically hypoxaemic patients may have exaggerated muscle oxidative stress, thus preventing a significant reduction in local muscle TNFα and IL‐6 mRNA expression.
Animal studies have shown that local muscle TNFα and IL‐6 expression impairs the ability of IGF‐I to promote protein synthesis10,34 and inhibits the expression of critical muscle‐specific transcription factors such as MyoD,35 resulting in skeletal muscle atrophy. It is therefore interesting to note that the significant training‐induced upregulation of both muscle IGF‐I and MyoD mRNA expression and changes in fibre morphological characteristics occurred despite the finding that muscle TNFα and IL‐6 mRNA expression was not significantly decreased after training. A possible explanation for these findings is that regular work overload is a powerful stimulus modulating an increase in factors that regulate myofibril hypertrophy, thereby exceeding the potentially negative impact of TNFα and IL‐6 on muscle architecture. On the other hand, exercise training in patients with chronic heart failure and healthy elderly subjects36,37 has been shown to decrease skeletal muscle TNFα mRNA expression and induce muscle fibre hypertrophy, thereby suggesting that training may reduce the inhibitory effect of TNFα on muscle protein synthesis. In chronic heart failure, exercise training has also been shown to reduce the local muscle mRNA expression of IL‐6.36 The duration of the training programme for 6 months in patients with chronic heart failure36 and/or the mode of exercise (resistance) in healthy elderly individuals37 may account for the differences between those two studies and the current study with regard to the effects of training on muscle inflammation.
In tandem with our previous findings,13 rehabilitative training induced a significant improvement in the cross‐sectional areas of all fibre types. Although it is known that the muscles of patients with COPD can hypertrophy in response to endurance training,11,12,13 the coupling to local growth factor expression has not previously been demonstrated. The present study is therefore the first to show that muscle fibre hypertrophy is accompanied by significant upregulation of IGF‐I (both at mRNA and protein levels) that is known to play an important role in the hypertophic adaptation of muscle to overload.17,18 The magnitude of the increase in IGF‐I mRNA expression (by ∼70%) in our COPD population is similar to that reported in patients with chronic heart failure after endurance training (by ∼80%),19 although it is lower than the increase reported after resistance training (by ∼300–500%) in healthy elderly subjects.17,18
Interestingly, the present study also showed a significant increase (by ∼70%) in the expression of one of the three isoforms of IGF‐I (ie, MGF) that is known to be sensitive to mechanical loading and to be upregulated after resistance training in healthy elderly individuals (increase in mRNA by ∼160%).15 It is therefore suggested that the muscles of patients with COPD are able to upregulate MGF mRNA in response to a period of endurance training.
The expression of MyoD is known to be highly induced after resistance training in healthy elderly individuals16 but, to the best of our knowledge, an increase in MyoD expression has not previously been reported after an endurance training programme in humans. MyoD is expressed in muscle satellite cells and mature myofibres and has been implicated in mediating the process of cell proliferation and differentiation for subsequent muscle regeneration and hypertrophy.38 Accordingly, it can be hypothesised that the repeated bouts of muscle loading within the present training programme caused significant upregulation of MyoD mRNA expression due to the activation of satellite cells for muscle repair/regeneration.38 The increase in local muscle MyoD protein levels was of a smaller magnitude than the mRNA expression. It has been suggested that, owing to a number of post‐transcriptional controls (eg, RNA splicing, RNA editing, blocked nuclear export, subcellular localisation, negative translational control), a close relationship between mRNA and protein would not be expected.9,39
Study limitations
The invasive nature of the muscle biopsies precluded a parallel, aged‐matched, healthy control training group which would allow comparisons in training‐induced changes in local muscle inflammatory and growth factors between healthy subjects and patients with COPD. However, in the study of Rabinovich et al,5 where such a control group was available, exercise training did not significantly modify local muscle TNFα mRNA in healthy subjects. In addition, another study40 involving endurance exercise training in healthy elderly individuals confirmed that this type of training does not impact on local muscle TNFα protein levels. Moreover, IGF‐I, MGF and MyoD mRNA levels have been shown to increase after resistance training in healthy subjects aged matched with those of our study.15,16,17
Although our previous work13 established no differences between training strategies in terms of muscle morphological characteristics, it is possible that the effectiveness of one of the two training modalities might be superior in terms of local muscle expression of growth and myogenic regulatory factors. However, in the present study there was not sufficient power within the two modalities to look for such differences. Future studies including larger sample size within each training group will allow such a comparison.
In conclusion, the results of the present study show that, in the absence of a decrease in systemic or local muscle TNFα and IL‐6, endurance exercise training in COPD induces peripheral muscle adaptations that are accompanied by upregulation of factors regulating skeletal muscle hypertrophy and regeneration.
Further information is given in the online supplement available at http://thorax.bmj.com/supplemental
Supplementary Material
Abbreviations
COPD - chronic obstructive pulmonary disease
CRP - C‐reactive protein
FEV1 - forced expiratory volume in 1 s
FVC - forced vital capacity
GAPDH - glyceraldehyde‐3‐phosphate dehydrogenase
IGF‐I - insulin‐like growth factor‐I
IL - interleukin
MGF - mechanogrowth factor
MyoD - myogenic differentiation factor D
TNFα - tumour necrosis factor α
Footnotes
This work was supported in part by the European Community CARED FP5 project (Contract No QLG5‐CT‐2002‐0893), the Thorax Foundation and by Pfizer Inc (Contract No GA9000K5).
Competing interests: None.
Further information is given in the online supplement available at http://thorax.bmj.com/supplemental
References
- 1.Agustí A G N. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 20052367–370. [DOI] [PubMed] [Google Scholar]
- 2.Broekhuizen R, Wouters E F M, Creutzberg E C.et al Elevated CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 20056117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosker H R, Wouters E F M, van der Vusse G J.et al Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr 2000711033–1047. [DOI] [PubMed] [Google Scholar]
- 4.Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J 200526703–719. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovich R A, Figueras M, Ardite E.et al Increased tumor necrosis factor‐α plasma levels during moderate‐intensity exercise in COPD patients. Eur Respir J 200321789–794. [DOI] [PubMed] [Google Scholar]
- 6.Montes de Oca M, Torres S H, De Sanctis J.et al Skeletal muscle inflammation and nitric oxide in patients with COPD. Eur Respir J 200526390–397. [DOI] [PubMed] [Google Scholar]
- 7.Koechlin C, Maltais F, Saey D.et al Hypoxaemia enhances peripheral muscle oxidative stress in chronic obstructive pulmonary disease. Thorax 200560834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broussard S R, McCusker R, Novakofski J E.et al Cytokine‐hormone interactions: tumor necrosis factor‐α impairs biologic activity and downstream activation signals of the insulin‐like growth factor I receptor in myoblasts. Endocrinology 20031442988–2996. [DOI] [PubMed] [Google Scholar]
- 9.Langen R C, Van Der Velden J L, Schols A M.et al Tumor necrosis factor‐alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 200418227–237. [DOI] [PubMed] [Google Scholar]
- 10.Haddad F, Zaldival F, Cooper D M.et al IL‐6‐induced skeletal muscle atrophy. J Appl Physiol 200598911–917. [DOI] [PubMed] [Google Scholar]
- 11.Debigare R, Cote C H, Maltais F. Peripheral muscle wasting in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 20011641712–1717. [DOI] [PubMed] [Google Scholar]
- 12.Whittom F, Jobin J, Simard P M.et al Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 1998301467–1474. [DOI] [PubMed] [Google Scholar]
- 13.Vogiatzis I, Terzis G, Nanas S.et al Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest 20051283838–3845. [DOI] [PubMed] [Google Scholar]
- 14.Bolton C E, Broekhuizen R, Ionescu A A.et al Cellular protein breakdown and systemic inflammation are unaffected by pulmonary rehabilitation in COPD. Thorax 200662109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hameed M, Lange K H W, Andersen J L.et al The effect of recombinant human growth hormone and resistance training on IGF‐I mRNA expression in the muscles of elderly men. J Physiol 2003555231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosek D J, Kim J, Petrella J K.et al Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 2006101531–544. [DOI] [PubMed] [Google Scholar]
- 17.Singh M A, Ding W, Manfredi T J.et al Insulin‐like growth factor‐I in skeletal muscle after weight‐lifting exercise in frail elders. Am J Physiol 1999277E135–E143. [DOI] [PubMed] [Google Scholar]
- 18.Kostek M C, Delmonico M J, Reichel J B.et al Muscle strength response to strength training is influenced by insulin‐like growth factor‐I genotype in older adults. J Appl Physiol 2005982147–2154. [DOI] [PubMed] [Google Scholar]
- 19.Hambrecht R, Schulze P C, Gielen S.et al Effects of exercise training on insulin‐like growth factor‐I expression in the skeletal muscle of non‐cachectic patients with chronic heart failure. Eur J Card Prev Rehab 200512401–406. [DOI] [PubMed] [Google Scholar]
- 20.Pauwels R A, Buist A S, Caverley P M.et al Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 20011631256–1276. [DOI] [PubMed] [Google Scholar]
- 21.Borg G A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 198214377–381. [PubMed] [Google Scholar]
- 22.Vogiatzis I, Nanas S, Kastanakis E.et al Dynamic hyperinflation and tolerance to interval exercise in patients with advanced COPD. Eur Respir J 200424358–363. [DOI] [PubMed] [Google Scholar]
- 23.Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J 20022012–19. [DOI] [PubMed] [Google Scholar]
- 24.Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF‐I mRNA content in human skeletal muscle. J Appl Physiol 2003951038–1044. [DOI] [PubMed] [Google Scholar]
- 25.Brooke M, Kaiser K. Muscle fiber types. How many and what kind? Arch Neurol 197023369–379. [DOI] [PubMed] [Google Scholar]
- 26.Papapetropulos A, Simoes D C M, Xanthou G.et al Soluble guanylyl cyclase expression is reduced in allergic asthma. Am J Physiol 2005277E135–E143. [DOI] [PubMed] [Google Scholar]
- 27.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 20043201043–1050. [DOI] [PubMed] [Google Scholar]
- 28.Barreiro E, Gea J, Corominas J M.et al Nitric oxide synthaseand protein oxidation in the quadriceps femoris of patientswith chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 200329771–778. [DOI] [PubMed] [Google Scholar]
- 29.Koechlin C, Couillard A, Cristol J P.et al Does systemic inflammation trigger local exercise‐induced oxidative stress in COPD? Eur Respir J 200423538–544. [DOI] [PubMed] [Google Scholar]
- 30.Debigare R, Marquis K, Cote C H.et al Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest 200312483–89. [DOI] [PubMed] [Google Scholar]
- 31.Fischer C P, Plomgaard P, Hansen A K.et al Endurance training reduces the contraction‐induced itereukin‐6 mRNA expression in human skeletal muscle. Am J Physiol 2004287E1189–E1194. [DOI] [PubMed] [Google Scholar]
- 32.Rabinovich R A, Ardide E, Troosters T.et al Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 20011641114–1118. [DOI] [PubMed] [Google Scholar]
- 33.Mercken E M, Hageman G J, Schols A M W J.et al Rehabilitation decrease exercise‐induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005164994–1001. [DOI] [PubMed] [Google Scholar]
- 34.Li Y P, Schwartz R J, Waddell I D.et al Skeletal muscle myocytes undergo protein loss and reactive oxygen‐mediated NF‐kΒ activation in response to tumor necrosis factor alpha. FASEB J 199812871–880. [DOI] [PubMed] [Google Scholar]
- 35.Costelli P, Muscaritoli M, Bossola M.et al Skeletal muscle wasting on tumor‐bearing rats is associated with MyoD down‐regulation. Int J Oncol 2005261663–1668. [DOI] [PubMed] [Google Scholar]
- 36.Gielen S, Adams V, Möbius‐Winkler S.et al Anti‐Inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 200342861–868. [DOI] [PubMed] [Google Scholar]
- 37.Greiwe J S, Cheng B, Rubin D C.et al Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 200115475–482. [DOI] [PubMed] [Google Scholar]
- 38.Charge S B, Rudnicki M A. Cellular and molecular regulation of muscle regeneration. Physiol Rev 200484209–238. [DOI] [PubMed] [Google Scholar]
- 39.Moore M J. From birth to death: the complex lives of eukaryotic mRNAs. Science 20053091514–1518. [DOI] [PubMed] [Google Scholar]
- 40.Ferrier K E, Nestel P, Taylor A.et al Diet but not aerobic exercise training reduces skeletal muscle TNF‐α in overweight humans. Diabetologia 200447630–637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.