Abstract
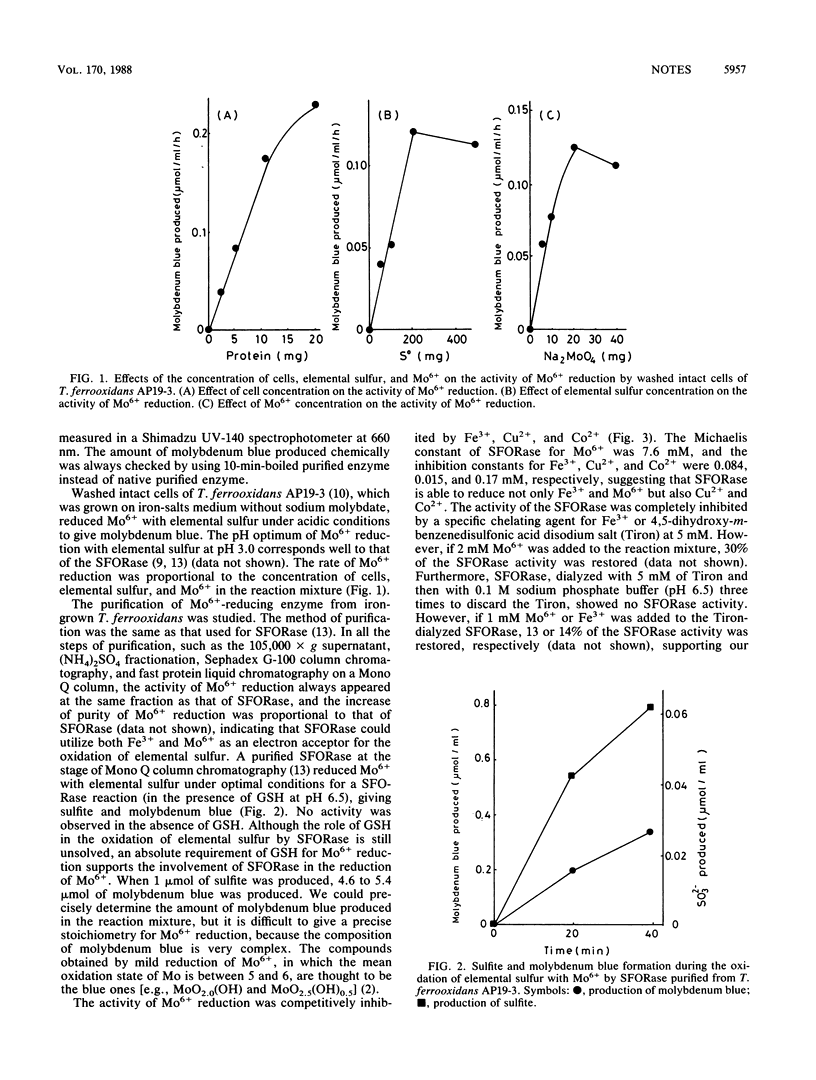
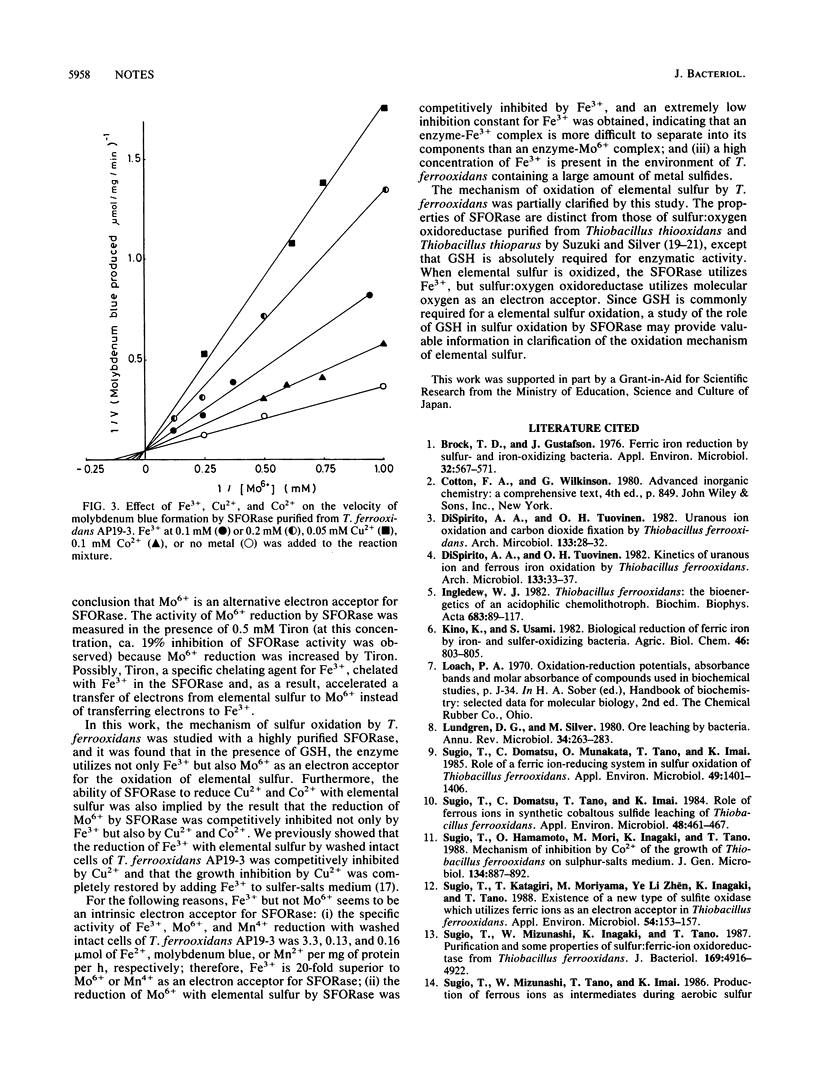
In the presence of phosphate ions, molybdic ions (Mo6+) were reduced enzymatically with elemental sulfur by washed intact cells of Thiobacillus ferrooxidans to give molybdenum blue. The whole-cell activity that reduced Mo6+ was totally due to cellular sulfur:ferric ion oxidoreductase (SFORase) (T. Sugio, W. Mizunashi, K. Inagaki, and T. Tano, J. Bacteriol. 169:4916-4922, 1987). The activity of M06+ reduction with elemental sulfur was competitively inhibited by Fe3+, Cu2+, and Co2+. The Michaelis constant of SFORase for Mo6+ was 7.6 mM, and the inhibition constants for Fe3+, Cu2+, and Co2+ were 0.084, 0.015, and 0.17 mM, respectively, suggesting that SFORase can reduce not only Fe3+ and Mo6+ but also Cu2+ and Co2+ with elemental sulfur.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D., Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol. 1976 Oct;32(4):567–571. doi: 10.1128/aem.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982 Nov 30;683(2):89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Lundgren D. G., Silver M. Ore leaching by bacteria. Annu Rev Microbiol. 1980;34:263–283. doi: 10.1146/annurev.mi.34.100180.001403. [DOI] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Munakata O., Tano T., Imai K. Role of a Ferric Ion-Reducing System in Sulfur Oxidation of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1985 Jun;49(6):1401–1406. doi: 10.1128/aem.49.6.1401-1406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Tano T., Imai K. Role of Ferrous Ions in Synthetic Cobaltous Sulfide Leaching of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1984 Sep;48(3):461–467. doi: 10.1128/aem.48.3.461-467.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Katagiri T., Moriyama M., Zhèn Y. L., Inagaki K., Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ions as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988 Jan;54(1):153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Mizunashi W., Inagaki K., Tano T. Purification and some properties of sulfur:ferric ion oxidoreductase from Thiobacillus ferrooxidans. J Bacteriol. 1987 Nov;169(11):4916–4922. doi: 10.1128/jb.169.11.4916-4922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio Tsuyoshi, Wada Kimihito, Mori Manami, Inagaki Kenji, Tano Tatsuo. Synthesis of an Iron-Oxidizing System during Growth of Thiobacillus ferrooxidans on Sulfur-Basal Salts Medium. Appl Environ Microbiol. 1988 Jan;54(1):150–152. doi: 10.1128/aem.54.1.150-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I. Incorporation of atmospheric oxygen-18 into thiosulfate by the sulfur-oxidizing enzyme of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Oct 25;110(1):97–101. doi: 10.1016/s0926-6593(65)80098-4. [DOI] [PubMed] [Google Scholar]
- Suzuki I. Oxidation of elemental sulfur by an enzyme system of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Jul 8;104(2):359–371. doi: 10.1016/0304-4165(65)90341-7. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Silver M. The initial product and properties of the sulfur-oxidizing enzyme of thiobacilli. Biochim Biophys Acta. 1966 Jul 6;122(1):22–33. doi: 10.1016/0926-6593(66)90088-9. [DOI] [PubMed] [Google Scholar]



