Abstract
Background
Vascular remodelling has recently been shown to be a promising pathogenetic indicator in idiopathic interstitial pneumonias (IIPs).
Aim
To validate the importance of the collagen/elastic system in vascular remodelling and to study the relationships between the collagen/elastic system, survival and the major histological patterns of IIPs.
Methods
Collagen/elastic system fibres were studied in 25 patients with acute interstitial pneumonia/diffuse alveolar damage, 22 with non‐specific interstitial pneumonia/non‐specific interstitial pneumonia and 55 with idiopathic pulmonary fibrosis/usual interstitial pneumonia. The Picrosirius polarisation method and Weigert's resorcin–fuchsin histochemistry and morphometric analysis were used to evaluate the amount of vascular collagen/elastic system fibres and their association with the histological pattern of IIPs. The association between vascular remodelling and the degree of parenchymal fibrosis in usual interstitial pneumonia (UIP) was also considered.
Results
The vascular measurement of collagen/elastic fibres was significantly higher in UIP than in the lungs of controls, and in those with diffuse alveolar damage and those with non‐specific interstitial pneumonia. In addition, the increment of collagen/elastic fibres in UIP varied according to the degree and activity of the parenchymal fibrosis. The most important predictors of survival in UIP were vascular remodelling classification and vascular collagen deposition.
Conclusion
A progressive vascular fibroelastosis occurs in IIP histological patterns, probably indicating evolutionarily adapted responses to parenchymal injury. The vascular remodelling classification and the increase in vascular collagen were related to survival in IIP and possibly play a role in its pathogenesis. Further studies are needed to determine whether this relationship is causal or consequential.
The vascular extracellular matrix (VECM) consists of collagens, elastin, fibrillins, proteoglycans and others.1 Collagen fibres are the most abundant component of the vascular pulmonary wall and are found in all three tunicae, especially around smooth muscle cells of the tunica media where they provide the necessary mechanical strength and contractility. Collagen fibres are also found in the outer layer (adventitia) where they form large bundles of fibrils which increase progressively in size from its innermost component, closest to the media, to its outermost aspect.2,3 The elastic system, a major component of the pulmonary arteries, plays an important role in wall elasticity, facilitated by concentric fenestrated lamellae of elastic fibres in the tunica media layer.1 The accumulation of ECM is an important process in pulmonary vascular structural remodelling.4,5 Elastosis has been well studied in animal models of pulmonary fibrosis and has been shown to be transcriptionally regulated by the augmentation of lytic enzymes.6 Studies previously conducted by our group showed that lung collagen and elastic fibre content are increased in both acute and chronic interstitial lung diseases, suggesting that significant remodelling of alveolar tissue occurs in both conditions.7,8,9,10,11 We also found evidence of vascular remodelling in lung biopsy specimens from patients with usual interstitial pneumonia (UIP); more specifically, a direct relationship between vascular regression—characterised by a progressive reduction in the internal area and internal perimeter, as well as by an increase in the wall thickness of medium or large lung vessels—and parenchymal remodelling.12 This finding is in agreement with previous reports of vessel ablation in areas of honeycomb lung, regardless of the cause of the pulmonary fibrosis.13,14,15,16 Experimental studies have shown that collagen and elastic fibre deposition might be important for understanding the alterations in vascular remodelling,17,18 but there has been uncertainty about the changes in the collagen/elastic system in lung vessels of patients with idiopathic interstitial pneumonias (IIPs).
We hypothesise that the collagen/elastic system in vascular remodelling differs according to the adaptive responses to injury that occur in acute interstitial pneumonia/diffuse alveolar damage (AIP/DAD), non‐specific interstitial pneumonia (NSIP)/NSIP and idiopathic pulmonary fibrosis (IPF/)/UIP.
This study was designed to measure the vascular collagen/elastic system in different adaptive responses to injury that occur in AIP/DAD, NSIP/NSIP and IPF/UIP, as well as their association with survival.
Methods
Patient selection
Pulmonary specimens were obtained by surgical lung biopsy from 109 patients, 25 with AIP/DAD, 22 with NSIP/NSIP and 55 with IPF/UIP, according to the criteria outlined in the American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the IIPs.19 Only specimens from patients who fulfilled these consensus criteria were included.
Specimens of any other possible aetiology (eg, pneumoconiosis) and/or with histological features suggestive of an alternative diagnosis (eg, eosinophilic pneumonia) were excluded, as were biopsy specimens obtained from patients with a concomitant systemic disease (eg, collagen vascular disease), extensive honeycomb changes (end‐stage lung disease), a dual histological pattern (two different patterns at two different biopsy sites), and/or morphological features not consistent with a specific histological pattern (eg, bronchocentric distribution in an otherwise classic case of UIP). After excluding specimens with histological and clinical evidence of desquamative interstitial pneumonia, lymphoid interstitial pneumonia or respiratory bronchiolitis, all patients included exhibited clinical, radiological and physiological changes consistent with AIP, NSIP or IPF and had been given the definitive pathological diagnosis of DAD, NSIP or UIP. Two or three biopsy specimens per patient were sampled and the tissue specimens collected according to the high‐resolution computed tomography (HRCT) pattern, which usually includes normal, intermediate and more affected areas in different parts of the lung. Thus, the diagnosis of our patients with IIPs was obtained by clinical, radiological and histological consensus criteria.
Baseline characteristics
The median age of patients with AIP (13 men, 12 women) was 51.7 years (range 35–74); 50.8 years (range 39–76) for NSIP (10 men, 12 women) and 65.3 years (range 50–84) for IPF (35 men, 20 women).
A baseline assessment of severity of dyspnoea was made using the Level of Dyspnoea Scale20 (table 1).
Table 1 Baseline characteristics of patients in the major histopathological categories.
| NSIP | AIP | IPF | |
|---|---|---|---|
| Age at biopsy (years) | 50.8 (16.6) | 51.7 (14.9) | 65.3 (7.4) |
| Sex (F/M) | 12/10 | 13/12 | 20/35 |
| Dyspnoea | |||
| LOD | 5 (4) | 14 (5) | 10 (4) |
| Spirometry | |||
| FEV1 (% pr) | 58 (6) | 60 (3) | 81 (5) |
| FVC (% pr) | 54 (5) | 51 (3) | 71 (4) |
| FEV1/FVC | 104 (7) | 97 (81) | 80 (6) |
| TLC (% pr) | 63 (5) | 57 (8) | 79 (4) |
| RV (% pr) | 94 (17) | 64 (15) | 93 (11) |
| RV/TLC (% pr) | 155 (16) | 93 (23) | 40 (3) |
| TLCO (% pr) | 43 (14) | 46 (9) | 60 (7) |
| TLCO/VA (% pr) | 97 (2) | 83 (5) | 63 (7) |
| Pao2 (kPa) | 8.5 (0.8) | 10.4 (2.4) | 7.9 (0.3) |
| Paco2 (kPa) | 4.7 (0.1) | 6.5 (0.7) | 4.4 (1.1) |
| HRCT Score | |||
| Alveolar | 1.6 (1.1) | 1.6 (0.9) | 1.87 (0.9) |
| Interstitial | 0.87 (0.61) | 0.73 (0.52) | 1.9(0.56) |
| Total | 2.47 (0.85) | 2.33 (0.71) | 3.77 (0.73) |
| Total CS | 33 (20) | 42 (23) | 53 (16) |
AIP, acute interstitial pneumonia; CS, clinical score; F, female; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HRCT, high‐resolution computed tomography; IPF, idiopathic pulmonary fibrosis; LOD, level of dyspnoea; M, male; NSIP, non‐specific interstitial pneumonia; Paco2, partial arterial pressure of carbon dioxide; Pao2, partial arterial pressure of oxygen; RV, residual volume; pr, predicted; TLC, total lung capacity; TLCO, carbon monoxide transfer factor; VA, alveolar volume.
Data are presented as means (SEM).
Physiological testing
The pulmonary function tests included forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio×100, total lung capacity (TLC), residual volume and carbon monoxide transfer factor (TLCO). TLC, residual volume and residual volume/TLC percentages were measured by the helium‐dilution method with a Master Screen Apparatus (Erich Jaeger GmbH, Wiirzburg, Germany), TLCO and TLCO/alveolar volume by the single breath‐holding helium‐dilution method.21 Lung function measurements (table 1) were expressed as percentages of predicted values. In all patients, the arterial Pao2 and Paco2 were also measured at rest. The cardiac parameters of these patients were normal.
High‐resolution computed tomography
HRCT examinations were performed using 1 or 1.5 mm‐thick sections taken at 1 cm intervals throughout the entire lung during inspirations in the supine position and through the caudal 10 cm of the lungs at 2–3 cm increments in the prone position. Two thoracic clinical radiologists prospectively and independently scored all lobes or HRCT scans for ground‐glass opacity (CT alveolar) and interstitial opacity (CT interstitial) on a scale of 0–5; the mean score for each lobe and for the entire lung was calculated22 (table 1).
Clinical scoring
Overall clinical severity was assessed using the previously developed Clinical and HRCT Examinations Composite Score.20 The total clinical and HRCT score ranges from 0 to 100 points (100 being the most severe disease) depending on variables including the Level of Dyspnoea Scale, HRCT and pulmonary function test results (table 1).
Pathological review of the specimens
Pulmonary specimens were reviewed by three pathologists blinded to their clinical features. Each specimen was assigned a histological diagnosis according to the criteria outlined in the American Thoracic Society/European Respiratory Society multidisciplinary consensus classification of the IIPs.19 DAD was characterised by involvement and a uniform temporal appearance caused by alveolar collapse, obliterative fibrosis, neosepta formation and moderately organising fibrosis.19 NSIP was characterised by temporally homogenous septal inflammatory thickening and minimal organising fibrosis.19 UIP was defined by alternating areas of normal parenchyma, alveolar collapse, honeycombing and severe organising fibrosis, defined as sites of active remodelling overlying fibrous airspace walls and thus showing temporal heterogeneity, or overlying normal rigid pulmonary structures (eg, interlobular septa) in the form of fibroblast foci and granulation tissue.19 Table 1 shows the demographic data and radiological characteristics.
As a control, normal lung tissues from non‐pneumonia and non‐emphysematous areas were obtained from five individuals (mean (SD) age 60 (3.6) years) who had died from violent causes. None of these controls met any of the histological criteria for IIP.
Morphological study
Parenchymal remodelling definition
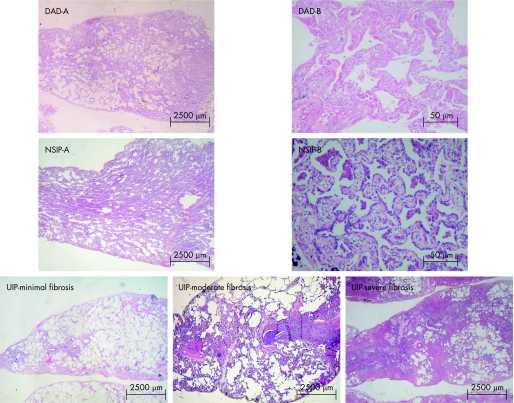
Parenchymal remodelling in UIP was evaluated by semiquantitative analysis for alternating areas of (1) minimal fibrosis (fig 1), defined as alveolar collapse with relatively unaffected lung tissue or with mild interstitial thickening by fibrosis; (2) moderate fibrosis (fig 1), defined by intermediate organising fibrosis of the wall with fibroblast foci; and (3) severe fibrosis (fig 1), defined as severe organising fibrosis of the wall with honeycombing and foci of actively proliferating fibroblasts and myofibroblasts.12
Figure 1 Idiopathic interstitial pneumonia (histological patterns). Diffuse alveolar damage (DAD)‐A. Panoramic view showing diffuse involvement and a uniform temporal appearance (H&E ×15); DAD‐B. High magnification showing areas of alveolar collapse, obliterative fibrosis and neoseptal formation (H&E ×400). Non‐specific interstitial pneumonia (NSIP)‐A. Characterised by temporally homogenous septal inflammatory thickening (H&E ×10). NSIP‐B. High magnification showing septal inflammatory thickening and moderate fibrosis (H&E ×400). Usual interstitial pneumonia (UIP)—minimal fibrosis. Characterised alveolar collapse with relatively unaffected lung tissue or with mild interstitial thickening by fibrosis (H&E ×10); UIP—moderate fibrosis. Pulmonary parenchyma showing a high degree of inflammatory activity and moderate mural organising fibrosis with fibroblast foci (H&E ×10); UIP—severe fibrosis. Severe mural organising fibrosis with honeycombing and foci of actively proliferating fibroblasts and myofibroblasts (H&E ×10)
Vascular remodelling definition
To study the possibility of an important vascular contribution to the fibrosis, we distinguished between vessels within the different areas of fibrosis and compared vessels from severely fibrotic areas with those in less fibrotic areas. In particular, vascular changes in relatively spared areas of patients with severe fibrosis were compared with similar areas in patients with less severe fibrosis to make a distinction between adventitia and surrounding parenchyma in patients with increasing fibrosis.
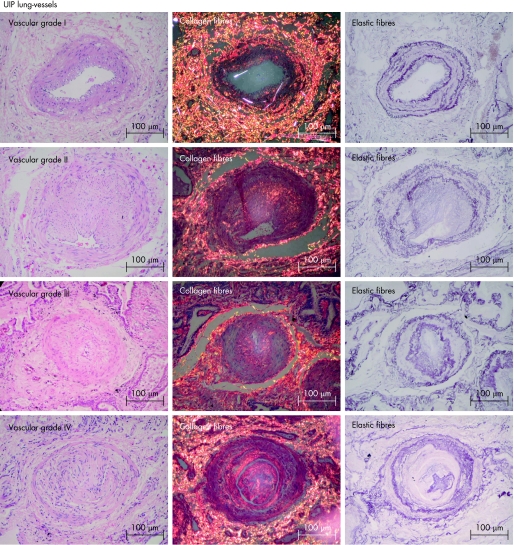
Vascular remodelling in UIP was initially evaluated by semiquantitative analysis for different levels of vascular obstruction in vessels within the different areas of fibrosis using a grading system as follows: grade I, isolated hypertrophy of the arterial media; grade II, proliferative intimal lesions; grade III, total occlusion of arterial lumen by fibrous tissue; and grade IV, plexiform lesions.
Collagen and elastic fibre density were then evaluated per biopsy specimen in 4–8 vessels with diameter ranging from 109.35 to 185.12 μm within the different areas of fibrosis. For the study of collagen, 3 μm paraffin wax‐embedded sections were stained in a 0.2% solution of Sirius red (Direct Red 80, CI 35780, Aldrich, Milwaukee, Wisconsin, USA) dissolved in aqueous saturated picric acid.23,24 Elastic staining was performed by Weigert's resorcin–fuchsin method after oxidation.25 The number of collagen/elastic fibres in artery walls was determined by an image analysis system in which a charge coupled device Sony DXC‐101 camera is coupled to a Zeiss Axioplan microscope, from which the images are sent to a monitor (Trinitron Sony). By means of a digitising system (Oculus TCX, Coreco; St Laurent, Quebec, Canada) inserted in a computer (Pentium 133 MHz), the images were processed by software (Bioscan‐Optimas 5.1; Bioscan, Edmonds, Washington, DC, USA). The enhancement of collagen birefringence promoted by the Picrosirius polarisation method is specific for collagenous structures composed of aggregates of orientated molecules. Elastic staining was performed by Weigert's resorcin–fuchsin method after oxidation. This method allows the selective identification of the three types of elastic system fibres (oxytalan, elaunin and fully developed elastic fibres). The thresholds for fibres of the collagenous and elastic systems were established for each slide after enhancing the contrast up to a point at which the fibres were easily identified as black (elastic) or birefringent (collagen) bands. The area occupied by the fibres was determined by digital densitometric reconiting, by adjusting the threshold level of measurement up to the grey density of the fibres of the collagenous and elastic systems. The collagen of the medial layer and the elastic fibre content were measured in each vascular wall and expressed as a relationship between the quantity of collagen and elastic fibres divided by the total vascular area studied. The vascular area of each artery analysed was carefully measured in the image analysis system using a cursor that allows the free determination of the area from the internal elastic membrane to the external elastic membrane. The results express the amount of fibres of the collagenous and elastic systems (in area) per total area of vascular wall expressed in fraction.
Statistical analysis
One‐way analysis of variance was used to analyse the variance in means of collagen and elastic fibre and their distribution in the histological pattern of IIPs (DAD, NSIP and UIP). Differences between the means were compared a priori by Levene's test for homogeneity of variance and then by post hoc tests using Bonferroni multiple comparisons for homogenous distribution and Dunnet T3 for non‐homogeneous distribution. Survival curves comparing the qualitative changes of the collagen and elastic fibres in the vascular wall and morphological parameters in NSIP, AIP and IPF were initially tested in a univariate model. The significant variables selected on the basis of a univariate model were considered in a Cox regression multivariate analysis using different model specifications. The level of significance was established at 0.05. The data were analysed using the SPSS for Windows program, release 10.0.26
Results
The different grades of vascular obstruction were significantly related to parenchymal changes in UIP compared with the histological patterns of DAD and NSIP. In other words, the alternating areas of minimal, moderate and severe fibrosis observed in UIP (fig 1) were significantly related to the degree of vascular occlusion (fig 2). High degrees of vascular occlusion (eg, grades III and IV) were correlated with a pulmonary parenchyma heavily compromised by fibrosis. In all UIP surgical lung biopsy specimens, alternating areas of moderate fibrosis were related to an intermediate degree of vascular remodelling or obstruction (grades II or III). In the remaining UIP lungs, categorised as alternating areas of minimal fibrosis, a significant relationship was found with a minimal degree of vascular remodelling—that is, vascular remodelling was represented by discrete intimal proliferation (grade I) with maintenance of the vascular architecture.
Figure 2 Usual interstitial pneumonia (UIP) lung vessels. This groups shows distortion of the vascular wall architecture and an increase in red–orange birefringence of collagen fibres in the vascular medial and adventitial layers in all four grades of vascular obstruction (grade I, isolated hypertrophy of the arterial media, grade II, proliferative intimal, grade III, total occlusion of the arterial lumen by fibrosis tissue and grade IV, plexiform lesion) and major proliferation of elastic fibres in the internal and external elastic lamina of the vascular wall (H&E, Picrosirius polarisation and Weigert's resorcin–fuchsin ×200).
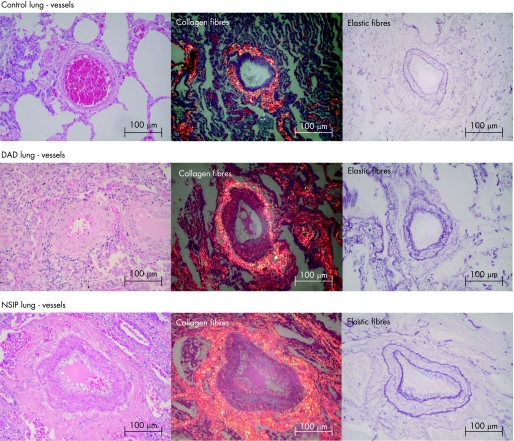
Figures 2 and 3 show the collagen/elastic fibre system in control and IIP lungs stained with Picrosirius polarised and Weigert's resorcin–fuchsin. Control lungs had weak red–orange birefringence in the adventitial tunica of the vascular wall in tissue sections and maintenance of the vascular wall architecture (fig 3). By contrast, UIP lung had distortion of the vascular wall architecture and an increase in birefringence in the vascular medial layer in all four degrees of vascular obstruction (fig 2). This correlated with an increase in parenchymal remodelling activity, septal thickening and increase in vascular collagen fibres (fig 1). DAD and NSIP had a moderate red–orange birefringence in the medial layers (fig 3). This increase in birefringence was greatest in NSIP lungs and correlated with moderate parenchymal remodelling and moderate alveolar septal thickening (fig 1). Equally important alterations of the elastic system were present. Figure 3 shows control groups in which the vascular pattern of the elastic component is preserved in the internal and external elastic lamina. Figure 2 shows major proliferation of elastic fibres in the internal and external elastic lamina of the vascular wall in UIP lungs, related to different degrees of vascular remodelling and different stages of parenchymal remodelling activity (fig 1). A moderate degree of elastic system proliferation was present in the vascular wall in the NSIP histological pattern whereas a minimal degree was observed in the DAD pattern (fig 3).
Figure 3 Control lung vessels showing collagen/elastic fibres in the adventitial tunica of the vascular wall in tissue sections and the maintenance of the vascular wall architecture (Picrosirius polarisation and Weigert's resorcin–fuchsin ×200). Diffuse alveolar damage (DAD) lung vessels showing a minimal red–orange birefringence of collagen fibres in the medial and adventitial layers and minimal proliferation of elastic fibres in the vascular wall (Picrosirius polarisation and Weigert's resorcin–fuchsin ×200). Non‐specific interstitial pneumonia (NSIP) lung vessels showing a moderate red–orange birefringence of collagen fibres and moderate proliferation of elastic fibres in the vascular wall (Picrosirius polarisation and Weigert's resorcin–fuchsin ×200).
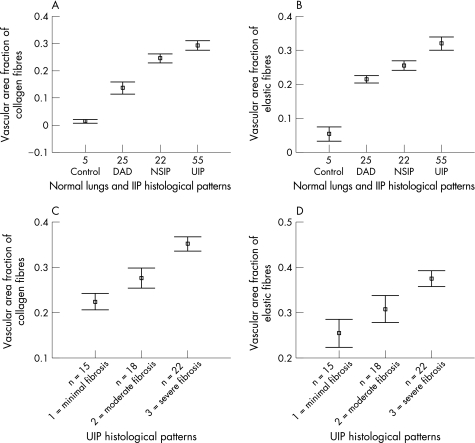
The qualitative changes in collagen and elastic fibres in the vascular wall correlated with differences in their quantification in the four groups of patients (table 2). The density of the collagen and elastic fibres was significantly higher in the artery walls of UIP lungs than in control NSIP and DAD lungs (p = 0.001). UIP vascular walls had the greatest amount of collagen, followed by NSIP, DAD and controls (fig 4A). A significant difference in the amount of collagen was observed among all groups (p = 0.001; fig 4A). Elastic fibres showed a similar pattern which was significant for control versus DAD (p<0.001); and NSIP versus UIP (p<0.001; fig 4A).
Table 2 Analysis of collagen and elastic vascular fibres stratified by histological patterns of idiopathic interstitial pneumonia (IIP) and control lung.
| Vascular collagen/elastic system | ||||
|---|---|---|---|---|
| Mean | SD | SE | 95% CI for mean | |
| Collagen fibres* | ||||
| Control | 0.01 | 0.005 | 0.002 | 0.007 to 0.02 |
| DAD | 0.13 | 0.05 | 0.01 | 0.11 to 0.15 |
| NSIP | 0.24 | 0.03 | 0.008 | 0.22 to 0.26 |
| UIP | 0.29 | 0.06 | 0.008 | 0.27 to 0.3 |
| Elastic fibres† | ||||
| Control | 0.05 | 0.01 | 0.0.7 | 0.03 to 0.07 |
| DAD | 0.21 | 0.02 | 0.005 | 0.20 to 0.22 |
| NSIP | 0.25 | 0.03 | 0.006 | 0.24 to 0.26 |
| UIP | 0.32 | 0.07 | 0.009 | 0.30 to 0.33 |
DAD, diffuse alveolar damage; IIP, idiopathic interstitial pneumonia; NSIP, non‐specific interstitial pneumonia; UIP, usual interstitial pneumonia.
Results analysed by the one‐way analysis of variance (ANOVA) procedure and Bonferroni multiple comparisons post hoc tests for ANOVA vascular collagen/elastic system fibres and their distribution in the histological patterns of IIP and control lung with level of significance 0.05.
*Control versus DAD, p<0.001; DAD versus NSIP, p<0.001, NSIP versus UIP, p = 0.009.
†Control versus DAD, p<0.001; DAD versus NSIP, p = 0.08; NSIP versus UIP, p<0.001.
Figure 4 Vascular area fraction of (A) collagen and (B) elastic fibres as a function of the control lungs compared with the histological patterns of diffuse alveolar damage (DAD), non‐specific interstitial pneumonia (NSIP) and usual interstitial pneumonia (UIP), and vascular area fraction of (C) collagen and (D) elastic fibres as a function of the histological patterns of UIP. IIP, idiopathic interstitial pneumonia. Results are shown as CI for means for each group.
Parenchymal remodelling in UIP ranged from minimal to moderate and severe fibrosis. In this group, the greater the increase in collagen and elastic fibres, the greater the parenchymal remodelling (fig 4C,D). The increase in fibre was greatest in lungs with parenchymal remodelling (severe fibrosis), while an intermediate increase in fibre was present in lungs with minimal and moderate fibrosis activity (p = 0.01; table 3) related to different degrees of vascular occlusion.
Table 3 Analysis of collagen and elastic vascular fibres stratified by usual interstitial pneumonia (UIP) histological patterns and control lung.
| Vascular collagen/elastic system | ||||
|---|---|---|---|---|
| Mean | SD | SE | 95% CI for mean | |
| Collagen fibres* | ||||
| Control | 0.01 | 0.05 | 0.002 | 0.07 to 0.02 |
| Minimal fibrosis | 0.22 | 0.03 | 0.008 | 0.2 to 0.24 |
| Moderate fibrosis | 0.27 | 0.04 | 0.01 | 0.25 to 0.29 |
| Severe fibrosis | 0.35 | 0.03 | 0.007 | 0.33 to 0.36 |
| Elastic fibres† | ||||
| Control | 0.05 | 0.01 | 0.007 | 0.03 to 0.07 |
| Minimal fibrosis | 0.25 | 0.05 | 0.01 | 0.22 to 0.28 |
| Moderate fibrosis | 0.3 | 0.05 | 0.01 | 0.27 to 0.33 |
| Severe fibrosis | 0.37 | 0.04 | 0.008 | 0.35 to 0.39 |
UIP, usual interstitial pneumonia.
Results analysed by one‐way analysis of variance (ANOVA) procedure and Dunnett T3 multiple comparisons post hoc tests for ANOVA vascular collagen/elastic system fibres and their distribution in the histological patterns of UIP and control lung with level of significance 0.05.
*Control versus UIP minimal fibrosis, p<0.001; UIP minimal fibrosis versus UIP moderate fibrosis, p = 0.02, UIP moderate fibrosis versus severe fibrosis, p = 0.0001.
†Control versus UIP minimal fibrosis, p = 0.001; UIP minimal fibrosis versus UIP moderate fibrosis, p = 0.037, UIP moderate fibrosis versus severe fibrosis, p = 0.01.
Fifty‐two patients died during the follow‐up period (5 NSIP, 12 AIP, 35 IPF). All patients studied had a restrictive lung function pattern characterised by a decrease in TLC (mean values were NSIP (63%), AIP (57%) and IPF (72%) of predicted values) and an increase in FEV1/FVC ratio×100 (mean values were NSIP (104%), AIP (97%) and IPF (83%) of predicted values). The mean predicted values of TLCO were decreased in patients with NSIP (43%), AIP (46%) and IPF (49%; table 1). Pulmonary function of patients with IPF with different degrees of fibrosis did not differ significantly between the groups (table 4). Pulmonary function tests were not significantly related to vascular occlusion or collagen and elastic vascular density.
Table 4 Baseline characteristics in the histological patterns of idiopathic pulmonary fibrosis.
| Variables | |||
|---|---|---|---|
| Minimal fibrosis | Moderate fibrosis | Several fibrosis | |
| Age at biopsy (years) | 62.2 (7.4) | 63.5 (8.6) | 67.5 (7.8) |
| Sex (F/M) | 5/10 | 7/11 | 8/14 |
| Dyspnoea | |||
| LOD | 8 (4) | 10 (2) | 12 (2) |
| Spirometry | |||
| FEV1 (% pr) | 74 (18) | 66 (20) | 90 (27) |
| FVC (% pr) | 66 (18) | 59 (17) | 76 (23) |
| FEV1/FVC | 87 (8) | 72 (34) | 90 (10) |
| TLC (% pr) | 67 (14) | 70 (84) | 76 (21) |
| RV (% pr) | 74 (15) | 78 (46) | 90 (46) |
| RV/TLC (% pr) | 43 (9) | 46 (17) | 44 (14) |
| TLCO (% pr) | 44 (17) | 54 (28) | 48 (22) |
| TLCO/VA(% pr) | 45 (17) | 52 (12) | 60 (20) |
| Pao2 (kPa) | 8.9 (1.7) | 8.4 (1.5) | 8.8 (1.9) |
| Paco2 (kPa) | 4.8 (0.7) | 4.8 (0.4) | 5.1 (0.5) |
| HRCT score | |||
| Alveolar | 1.7 (0.22) | 1.75 (0.4) | 1.8 (0.5) |
| Interstitial | 1.85 (0.61) | 1.87 (0.42) | 1.89 (0.56) |
| Total | 3.55 (0.83) | 3.52 (0.82) | 3.69 (1.06) |
| Total CS | 50 (20) | 52 (26) | 53 (12) |
CS, clinical score; F, female; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HRCT, high‐resolution computed tomography; IPF, idiopathic interstitial pneumonia; LOD, level of dyspnoea; M, male; Paco2, partial arterial pressure of carbon dioxide; Pao2, partial arterial pressure of oxygen; RV, residual volume; pr, predicted; TLC, total lung capacity; TLCO, carbon monoxide transfer factor; VA, alveolar volume.
Data are presented as means (SEM).
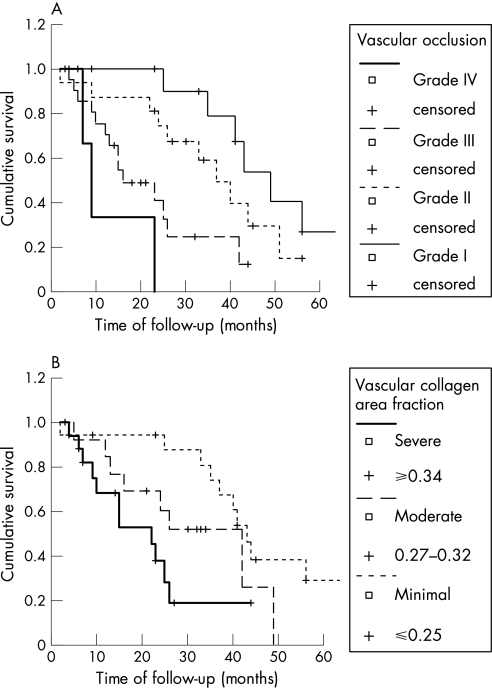
In the first statistical test, the individual effect of patient characteristics (age, sex, baseline data and physiological test), parenchymal remodelling (minimal fibrosis, moderate fibrosis and severe fibrosis) and vascular remodelling (grade I, grade II, grade III, grade IV, collagen and elastic fibre density) were examined to estimate the survival curve (fig 5A,B). The results of this analysis showed that the prognosis of patients with IPF/UIP was dependent on the vascular remodelling classification (log rank 18.24; p<0.001; fig 5A) and the collagen vascular density (log rank 10.57; p = 0.005; fig 5B). Multivariate analysis of overall survival time based on significant factors at univariate analysis was examined by the Cox regression model. Initially, the model was constructed with vascular remodelling classification and collagen vascular density. In this situation, only the vascular remodelling classification was maintained as an independent prognostic factor; collagen vascular density lost significance probably owing to the joint effects of both vascular variables (table 5). Thus, total occlusion of the arterial lumen by fibrous tissue (grade III) and plexiform lesions (grade IV) increases the risk of death in patients with UIP/IPF by 4–11‐fold.
Figure 5 Kaplan–Meier survival curve for patients with idiopathic pulmonary fibrosis/usual interstitial pneumonia (IPF/UIP) grouped by (A) vascular classification system (p<0.01) and (B) vascular area fraction of collagen (p<0.01).
Table 5 Cox multivariate analysis coefficients relating the amount of collagen fibres, elastic fibres and vascular occlusion classification to overall survival in idiopathic interstitial pneumonia/usual interstitial pneumonia.
| Vascular variables | B | SE | Wald | Sig | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Collagen fibres | |||||||
| ⩽0.25 | 0.638 | 0.727 | |||||
| 0.27–0.32 | −0.093 | 0.770 | 0.015 | 0.904 | 0.911 | 0.201 to 4.12 | |
| ⩾0.34 | 0.314 | 0.843 | 0.139 | 0.710 | 1.369 | 0.262 to 7.145 | |
| Elastic fibres | |||||||
| ⩽0.27 | 0.407 | 0.816 | |||||
| 0.29–0.35 | 0.391 | 0.618 | 0.401 | 0.527 | 1.479 | 0.440 to 4.969 | |
| ⩾0.36 | 0.282 | 0.695 | 0.165 | 0.685 | 1.326 | 0.340 to 5.176 | |
| Vascular occlusion | |||||||
| Grade I | 4.815 | 0.186 | |||||
| Grade II | 0.590 | 0.584 | 1.021 | 0.312 | 1.804 | 0.574 to 5.664 | |
| Grade III | 1.397 | 0.714 | 3.826 | 0.050 | 4.045 | 0.997 to 16.406 | |
| Grade IV | 2.408 | 1.320 | 3.329 | 0.068 | 11.113 | 0.836 to 147.667 |
B, Cox's regression coefficient; Exp, exponent; Sig, significance.
Discussion
The probable reason why patients with IIPs have different outcomes is uncontrolled fibrosis progression. The question of interest is whether further information gathered from the lung parenchyma or interstitial or VECM active remodelling can help us understand the heterogeneous development of IIPs. The process of active remodelling comprises a series of complex sequential steps, of which ECM remodelling is thought to be important because this process facilitates scar formation which occurs by neovascularisation, myofibroblast migration, and collagen and elastic fibre deposition. In IIPs, this process takes place in an uncontrolled way, causing excessive fibrosis formation and progressive histoarchitectural and vascular changes.27,28,29,30,31,32,33 VECM remodelling is a dynamic process that involves changes in collagen and elastic fibres resulting in different degrees of vascular occlusion. Collagen fibres are distributed diffusely throughout the media and adventitia of vessels, being synthesised by fibroblasts, myofibroblasts and smooth muscle cells, and providing tensile strength to the vessel wall.34 Elastic fibres are composed of an amorphous component, called elastin, and a highly structured microfibrillar non‐extensible component.17,18 In normal elastic arteries, the internal and external elastic laminas are composed mainly of fully mature elastic fibres that confer a high degree of elasticity to the vascular tissue.17,18 Owing to their mechanical properties, elastic fibres provide the elasticity needed for good vascular function.17,18 Increased elastin destruction takes place under certain pathological conditions due to the release of powerful elastolytic proteases by inflammatory cells.35 Reactivation of elastin synthesis is observed in response to the increased destruction,36 but in a highly disordered manner with deleterious consequences to the vascular mechanical properties.37
We should therefore not be surprised to learn that vascular changes provide important information about the active remodelling in IIPs, and our results confirm the importance of vascular remodelling in DAD, NSIP and UIP, the most important types of IIPs. Although only two previous studies were able to show a significant relationship between pathological and structural vascular changes in pulmonary models,37,38,39,40 our results suggest that increases in collagen and elastic fibres in vessels probably contribute to vascular changes in fibrotic lung disease. There is no evidence in our study that these fibrotic changes can be attributed to any form of pulmonary hypertension. In contrast, changes in the vessels in fibrotic lung disease seem to be secondary to the inflammatory changes and are therefore not the result of increased vascular flow but rather the result of the effects of cytokines and other mediators on myofibroblasts and smooth muscle cells, also with possible endothelial damage and thrombosis. The examples given in the figures also support the fact that these are not consistent with changes generally seen in different stages of plexiform arteriopathy, as observed in pulmonary hypertension. The use of a grading system as described in our study was therefore able to discern different levels of severity of obstruction.
UIP was shown to be the prototype of apposition of elastic and collagen fibres in the vascular wall. We found a correlation between active vascular remodelling measured by vascular classification, vascular grades of collagen deposition and survival in IPF/UIP. Although we were unable to determine whether vascular remodelling is a primary event or whether it is secondary to interstitial fibrosis, increases in collagen/elastic system fibres enable us to follow the development of fibrosis in patients with UIP. Our results require further study in randomised and prospective trials, and it is important to validate our quantitative assessment of collagen/elastic fibres as well as to extend it to other diffuse parenchymal lung diseases by studying ECM remodelling in more patients.
We have also found that the amount of collagen/elastic fibres was related to different forms of remodelling in NSIP and DAD. For instance, collagen/elastic fibre density in DAD and NSIP was significantly related to the histological pattern, and collagen/elastic fibre quantification provides more information about the remodelling state. The amount of collagen/elastic fibre was also significantly related to the responses to VECM remodelling, which depend largely, if not solely, on the extent of the destruction of the lung parenchyma as currently seen in DAD and NSIP. Most interesting was the progressive increase seen between the collagen/elastic system and DAD, NSIP and UIP. Collagen/elastic fibres increased progressively from DAD and NSIP to UIP, emphasising a temporal increase in IIPs, where fibre deposition and parenchymal activity were considered intermediate between UIP and DAD. In the collagen and elastic vascular area, the adventitial area was not included and this area was carefully delineated to avoid areas with increasing interstitial fibrosis in the lung. The distinction between the fibrotic part of the adventitia and the surrounding parenchyma was difficult.
A number of associations between vascular remodelling and IIPs have recently been described.41 Increases in collagen and elastic fibres in arteries are thought to determine contracture and reduced distensibility of the vessels in IIPs, thus causing chronic hypoxia, and they are also thought to increase according to parenchymal injury extension. More specifically, we found a direct relationship between vascular remodelling and the degree of parenchymal fibrosis in UIP. Peao et al13 also showed that vascular remodelling regulates lung fibrosis induced by bleomycin in rats. In 1963, Turner‐Warwick16 reported precapillary systemic–pulmonary anastomoses as part of the vascular remodelling in lungs. In addition, these findings are also in agreement with previous reports of vessel ablation in areas of honeycomb lung, regardless of the cause of pulmonary fibrosis. In this study we have shown that there is a strong correlation between vascular remodelling and levels of collagen/elastic fibre deposition in UIP which, to the best of our knowledge, is the first report of this association in IIPs. The strong association between vascular collagen/elastic fibre remodelling and IIPs suggests that higher levels of collagen/elastic fibres are secreted in IIPs which facilitate parenchyma destruction and ECM fibroelastosis. Thus, increased collagen/elastic fibre levels may be a primary event and increased vascular remodelling may be a secondary event. Regardless of the mechanism, the collagen/elastic system provides important information in IIPs.
During the follow‐up of the 102 patients, 52 died (clinical diagnoses: NSIP (n = 5), AIP (n = 12) and IPF (n = 35)). The survival analysis showed that the prognosis of patients with IPF/UIP was dependent on vascular remodelling classification and the collagen vascular density. However, when multivariate analysis of overall survival time was examined by the Cox regression model, only vascular remodelling classification remained an independent prognostic factor; collagen vascular density lost significance probably because of the joint effects of both vascular variables. Thus, total occlusion of the arterial lumen by fibrous tissue (grade III) and plexiform lesions (grade IV) increases the risk of death for patients with UIP/IPF by 4–11‐fold.
In conclusion, vascular remodelling present in IIPs differs in terms of collagen and elastic fibre content. This vascular fibroelastosis correlates with the degree of fibrosis. This suggests that vascular remodelling in DAD, NSIP and UIP runs in parallel with the adaptive responses after injury in these entities which depend, at least in part, on the extent of collagen and elastic fibre deposition. In UIP lungs, vascular occlusion is related to survival and may have an important role in disease pathogenesis.
Abbreviations
AIP - acute interstitial pneumonia
DAD - diffuse alveolar damage
ECM - extracellular matrix
FEV1 - forced expiratory volume in 1 s
FVC - forced vital capacity
HRCT - high‐resolution computed tomography
IIP - idiopathic interstitial pneumonia
IPF - idiopathic pulmonary fibrosis
NSIP - non‐specific interstitial pneumonia
UIP - usual interstitial pneumonia
VECM - vascular extracellular matrix
Footnotes
Funding: This study was supported by the following Brazilian agencies: the National Council for Scientific and Technological Development (CNPq); the Foundation for the Support of Research of the State of São Paulo (FAPESP 2001/14566‐9); and the Laboratories for Medical Research (LIM 05) Clinicas Hospital, School of Medicine, University of São Paulo, São Paulo, Brazil.
Competing interests: None declared.
References
- 1.Godwin T A.Respiratory system. Department of Pathology, New York Hospital Queens USA 2005
- 2.Kehrel B. Platelet‐collagen interactions. Semin Thromb Hemost 199521123–129. [DOI] [PubMed] [Google Scholar]
- 3.Lethias C, Labourdette L, Willems R.et al Composition and organization of the extracellular matrix of vein walls: collagen networks. Int Angiol 199615104–113. [PubMed] [Google Scholar]
- 4.Dzau V J, Gibbons G H. Vascular remodeling: mechanisms and implication. J Cardiovasc Pharmacol 199321(Suppl)S1–S5. [PubMed] [Google Scholar]
- 5.Gibbons G H, Dzau V J. The emerging concept of vascular remodeling. N Engl J Med 19943301431–1438. [DOI] [PubMed] [Google Scholar]
- 6.Leick‐Maldonado E A, Lemos M, Tibério I F L C.et al Differential distribution of elastic system fibers in control and bronchoconstricted intraparenchymatous airways in the guinea‐pig lung. J Submicrosc Cytol Pathol 199729427–434. [PubMed] [Google Scholar]
- 7.Saldiva P H N, Capelozzi V, Carvalho C R R.et al Histochemical evaluation of lung collagen content in acute and chronic interstitial diseases. Chest 198995953–957. [DOI] [PubMed] [Google Scholar]
- 8.Negri E M, Montes G S, Saldiva P H N.et al Architectural remodelling in acute and chronic interstitial lung disease: fibrosis or fibroelastosis. Histopathology 200037393–401. [DOI] [PubMed] [Google Scholar]
- 9.Negri E M, Hoelz C, Barbas C S V.et al Acute remodelling of parenchyma in pulmonary and extrapulmonary ARDS. An autopsy study of collagen‐elastic system fibers. Pathol Res Pract 2002198355–361. [DOI] [PubMed] [Google Scholar]
- 10.Faffe D S, Silva G H, Kurtz P M.et al Lung tissue mechanics and extracellular matrix composition in a murine model of silicosis. J Appl Physiol 2001901400–1406. [DOI] [PubMed] [Google Scholar]
- 11.Rocco P R M, Negri E M, Kurtz P M.et al Lung tissue mechanics and extracellular matrix remodelling in acute lung injury. Am J Respir Crit Care Med 20011641067–1071. [DOI] [PubMed] [Google Scholar]
- 12.Parra E R, David Y R, da Costa L R S.et al Heterogeneous remodeling of lung vessels in idiopathic pulmonary fibrosis. Lung 2005183291–300. [DOI] [PubMed] [Google Scholar]
- 13.Peao M N D, Aguas A P, DeSa C M.et al Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat Rec 199423857–67. [DOI] [PubMed] [Google Scholar]
- 14.Renzoni E A, Walsh D A, Salmon M.et al Interstitial vascularity in fibrosing alveilitis. Am J Respir Crit Care Med 2003167438–443. [DOI] [PubMed] [Google Scholar]
- 15.Salmon M, Lui Y C, Mark J C.et al Contribution of upregulation airway endothelin –1 expression to airway smooth muscle and epithelial cell DNA synthesis after repeated allergen exposure of sensitized Brown‐Norway rats. Am J Respir Cell Mol Biol 200023618–625. [DOI] [PubMed] [Google Scholar]
- 16.Turner‐Warwick M. Precapillary systemic‐pulmonary anastomoses. Thorax 196318225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karnik S K, Brooke B S, Bayes‐Genis A.et al A critical role for elastin signaling in vascular morphogenesis and disease. Development 2003130411–423. [DOI] [PubMed] [Google Scholar]
- 18.Li, DY Elastin is an essential determinant of arterial morphogenesis. Nature 1998393276–280. [DOI] [PubMed] [Google Scholar]
- 19.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J 200219794–796. [DOI] [PubMed] [Google Scholar]
- 20.Watters L C, King T E, Schwarz M I.et al A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis 198613397–103. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PhH, Tammeling G J, Cotes J E.et al Lung volumes and forced ventilatory flows. Report working party, Standardization of lung function tests, European Community for steel and coal. Official Statement of the European Respiratory Society. Eur Respir J 19936(Suppl 16)5–40. [PubMed] [Google Scholar]
- 22.Kazerooni E A, Martinez F J, Flint A.et al Thinsection CT obtained at 10 mm increments versus three‐level thin‐section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. Am J Roentgenol 1997169977–983. [DOI] [PubMed] [Google Scholar]
- 23.Montes G S, Junqueira L C U. Histochemical localization of collagen and of proteoglycans in tissues. In: Nimni ME, ed. Collagen Vol 2. Boca Raton, FL: CRC Press, 199841–72.
- 24.Montes G S. Structural biology of the fibers of the collagenous and elastic systems. Cell Biol Intermed 199620245–249. [DOI] [PubMed] [Google Scholar]
- 25.Lemos M, Pozo R M K, Montes G S.et al Organization of collagen and elastic fibers studied in stretch preparations of whole mounts of human visceral pleura. Ann Anat 199779447–452. [DOI] [PubMed] [Google Scholar]
- 26.Norusis M J. SPSS for Windows. [10.0]. Chicago: SPSS, 2001
- 27.Basset F, Ferrans V J, Soler P.et al Intraluminal fibrosis in interstitial lung disorders. Am J Pathol 1986122443–461. [PMC free article] [PubMed] [Google Scholar]
- 28.Karnik S K, Brooke B S, Bayes‐Genis A.et al A critical role for elastin signaling in vascular morphogenesis and disease. Development 2003130411–423. [DOI] [PubMed] [Google Scholar]
- 29.Myers J L, Katzeinstein A L. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest 1988941309–1311. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda Y, Basset F, Soler P.et al Intraluminal fibrosis and elastic fiber degradation lead to lung remodelling in pulmonary Langerhans cell granulomatosis (histiocytosis X). Am J Pathol 1990137415–424. [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuda Y M, Ishizaki M, Kudoh S.et al Localization of matrix metalloproteinases‐1, ‐2, and ‐9 and tissue inhibitor of metalloproteinase‐2 in interstitial lung diseases. Lab Invest 199878687–698. [PubMed] [Google Scholar]
- 32.Fukuda Y, Ishizaki M, Masuda Y.et al The role of intra‐alveolar fibrosis in the process of pulmonary structural remodelling in patients with diffuse alveolar damage. Am J Pathol 1987126171–182. [PMC free article] [PubMed] [Google Scholar]
- 33.Barbas Filho J V, Ferreira M A, Sesso A.et al Evidences of type II pneumocytes apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP). J Clin Pathol 200154132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tozzi C A, Christiansen D L, Poiani G J.et al Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am J Respir Crit Care Med 19941491317–1326. [DOI] [PubMed] [Google Scholar]
- 35.Bitterman P B, Pollunovsky V A, Ingbar D H. Repair after acute lung injury. Chest 1994105(Suppl 118)120. [DOI] [PubMed] [Google Scholar]
- 36.Mariani T J, Crouch E, Rouby J D.et al Increased elastin production in experimental granulomatous lung disease. Am J Pathol 1995147988–1000. [PMC free article] [PubMed] [Google Scholar]
- 37.Li D Y. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol 199761021–1028. [DOI] [PubMed] [Google Scholar]
- 38.Ried L, Meyrick B. Hypoxia and pulmonary vascular endothelium. Ciba Fund Symp 19807837–60. [DOI] [PubMed] [Google Scholar]
- 39.Meyrick B, Reid L. Hypoxia‐induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol 1980100151–178. [PMC free article] [PubMed] [Google Scholar]
- 40.Vyas‐Somani A C, Aziz S M, Arcot S A.et al Temporal alterations in basement membrane components in the pulmonary vascular of the chronically hypoxic rat: impact of hypoxia and recovery. Am J Med Sci 199631254–67. [DOI] [PubMed] [Google Scholar]
- 41.Masahito E, Minoru S, Naoko S.et al Heterogeneous increase in CD34‐positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 20041691203–1208. [DOI] [PubMed] [Google Scholar]