Abstract
Background
Monitoring of respiratory function is important in the diagnosis and management of respiratory disease. The forced oscillation technique requires minimal patient cooperation and is ideal for the determination of respiratory function in young children. This study aimed to develop reference ranges and to document the repeatability in healthy young children using commercially available forced oscillation equipment.
Methods
The forced oscillation technique, which uses a pseudo‐random noise forcing signal between 4 and 48 Hz, was used to measure respiratory function in healthy young children. Repeatability over a 15 min period was also assessed. Regression equations and standardised Z scores were determined for respiratory resistance (Rrs) and reactance (Xrs) at 6, 8 and 10 Hz.
Results
Respiratory function was obtained in 158 healthy children aged two to seven years and between 92 and 127 cm in height. Oscillatory respiratory mechanics exhibited linear relationships with height. Within‐test variability for resistance ranged between 6% and 9% and between 17% and 20% for reactance. Resistance and reactance did not change significantly over a 15 min period.
Conclusions
Reference ranges for respiratory impedance variables in healthy children aged two to seven years are presented. The short‐term repeatability of forced oscillatory variables in this age group is reported, allowing appropriate cut‐off values for therapeutic interventions to be defined.
Respiratory function measurements are important in the diagnosis and management of respiratory disease. In adults and older children, spirometry is the most commonly performed respiratory function test, but reliable and reproducible spirometric results may be difficult to obtain in young children. The forced oscillation technique (FOT) requires minimal patient cooperation and is ideally suited to subjects unable to perform voluntary forced expirations. The FOT has been used in research studies for several decades and, more recently, its use in clinical practice has been advocated, as reviewed by Oostveen and colleagues.1
Before the introduction of the FOT into clinical practice, it is imperative that appropriate reference values derived from a healthy population are available. The reference population should be of similar anthropometric distribution to the clinical population to be tested, and the equipment and methods used to obtain the clinical data should be comparable to the equipment and methods used to collect the reference data.2 Forced oscillatory mechanics in healthy pre‐school age children using pseudo‐random frequency,3,4,5 fixed frequency6,7,8,9 or impulse generator10,11,12,13 FOT equipment have been reported. Studies with significant numbers of young children, however, are less common and the majority of recent publications using commercial equipment use an impulse oscillation system.10,11,12,13 To our knowledge, reference data for commercially available FOT equipment utilising pseudo‐random forcing signals between 4 and 48 Hz (I2M, Chess Medical, Ghent, Belgium) do not exist. The majority of earlier studies using pseudo‐random forcing signals to obtained forced oscillatory mechanics in healthy young children have reported respiratory resistance (Rrs) data alone, and relationships of respiratory reactance (Xrs) with body size are not well characterised with this form of equipment. Lack of standardisation of the measurement, forcing signal, analysis and reporting for forced oscillatory studies has also resulted in a wide range of reported values of Rrs at a given age.1
The characterisation of between‐test repeatability is an important component of the application of a technique, particularly in younger children in whom respiratory function or ability to perform respiratory function tests may be more variable. Studies reporting between‐test repeatability of the FOT in young children have used impulse oscillations,10,11,12 and we are not aware of studies documenting repeatability of the FOT obtained using pseudo‐random forcing signals. Such information is required so that results following interventions (eg, bronchodilator responsiveness) can be interpreted appropriately.
The purpose of this study was to:
Characterise forced oscillatory mechanics using commercially available equipment using a broad frequency spectrum in a population of healthy young children and thus provide a basis for future studies in young children with respiratory disease.
Document repeatability data of FOT variables to ensure valid interpretation of changes following therapeutic or diagnostic interventions.
Methods
Subjects
Cross‐sectional study of respiratory function in healthy young children
One hundred and fifty‐eight healthy children (74 boys) aged two to seven years recruited for various research protocols were identified. Anthropometric data are summarised in table 1. All protocols included the assessment of respiratory system input impedance (Zrs) using the FOT as described below. Respiratory history was assessed using an administered questionnaire and children were classed as healthy if they had no history of doctor‐diagnosed or parentally‐reported respiratory disease, wheeze or asthma at any time in their life. Children with a parent‐reported history of recurrent cough were excluded only if they had cough in the last 12 months. Atopy was determined by skin prick testing to nine common aero‐allergens in 136 of the children. The study received ethical approval from the Princess Margaret Hospital Human ethics committee and parents gave written consent for their children to participate.
Table 1 Anthropometric data of study subjects.
| Healthy group (n = 158) | Repeatability (n = 69) | |
|---|---|---|
| History (H/W/NA) | 158/0/0 | 35/30/5 |
| Height (cm) | 110 (101.6–119.0) | 114 (105–124) |
| Weight (kg) | 19.4 (15.86–23.21) | 21 (17.0–27.50) |
| Age (years) | 5.08 (3.82–6.07) | 5.75 (4.67–6.67) |
H, healthy; W, history of wheeze ever; NA, history not available.
Continuous data expressed as median (10–90th centiles).
Short‐term repeatability
Seventy children (36 boys) participating in a field study were recruited for the measurement of short‐term repeatability (table 1). Oscillatory measurements were conducted at the child's school. One child refused the measurement resulting in paired measurements being attempted in 69 children (35 boys). Not all of those subjects recruited for the assessment of short‐term repeatability were classed as healthy (as defined above), and only data obtained from healthy children (n = 33) were included in the broader analysis of relationships between oscillatory mechanics and body size.
Protocols
Children were studied when well and without recent respiratory tract infections. They sat in an upright position with their head in a neutral position and were connected to the oscillation device via a mouthpiece incorporating a bacterial filter (SureGard; BirdHealthcare, Melbourne, Australia) and instructed to breathe normally with a noseclip in place. The cheeks and lower jaw of the subject were firmly supported by a staff member during all measurements.
To characterise the short‐term repeatability of the FOT, two measurement sets were obtained approximately 15 min apart.
Forced oscillatory measurements
Measurements of Zrs were obtained using a commercially available device (I2M, Chess Medical) and performed according to European Respiratory Society recommendations.1 The equipment accuracy was verified daily using a known resistance. Measured Zrs spectra were corrected for the impedance characteristics of the mouthpiece. This commercial FOT device is based on the research equipment prototype described by Landser et al.14 Briefly, a loudspeaker‐generated pseudo‐random noise forcing signal containing integer‐multiple frequencies between 2 and 48 Hz was applied at the mouth of the child. The low amplitude forcing signal is constructed so as to bias the energy content to the lower frequencies, with the relative energy content decreasing exponentially with frequency. Mouth pressure and flow were recorded for 8 s per measurement at the airway opening using identical piezoresistive differential pressure transducers (pressure range 1 psi, frequency response 1 ms/1000 Hz; IC Sensors, 1220 Series, Measurement Specialities, USA). Data were sampled at 128 Hz and a fast Fourier transform performed with a moving window average of 1024 samples. The Zrs spectra are calculated from both the inspiratory and expiratory signals. The coherence function, which is a measure of linearity of the signal, was calculated at each frequency.14
Minor variations in the number of Zrs measurements collected occurred between populations. Those children participating in the field study had a set number of five Zrs measurements attempted without the patient disconnecting from the equipment. All other children had a minimum of five and a maximum of 10 Zrs measurements collected and were allowed to disconnect from the equipment between measurements if desired.
In all cases, individual measurements were excluded if any of the following were noted: incomplete seal around the mouthpiece (leak), movement of mouth (chewing), swallowing, glottal closure, talking or audible noise. Individual Zrs measurements were also examined post hoc for evidence of inadequate measurement quality at specific frequencies. Measurement quality was defined as being acceptable if the value of the coherence function at an individual frequency was ⩾0.95,15 and measurements in which three or more individual frequencies had a coherence of <0.95 were excluded.
The following Zrs variables were analysed and reported for each child: Rrs and Xrs at 6, 8 and 10 Hz (Rrs6, Rrs8, Rrs10, Xrs6, Xrs8 and Xrs10, respectively). Individual data are reported as the mean of all technically acceptable measurements, with the caveat that children with less than three acceptable Zrs measurements were excluded from further analysis. The average Rrs between 4 and 24 Hz (Rrs4–24) and the frequency dependence (Fdep) of Rrs4–24 characterised as the slope of Rrs4–24 plotted against frequency (Fdep: (Rrs24–Rrs4)/20) were calculated from the best fit polynomial of the average Rrs data between 4 and 24 Hz.
Statistics
Data are expressed as mean (SE) or as median (10–90th centiles) for normal and non‐normal distributions, respectively. Individual within‐test variations for Zrs parameters are expressed as coefficients of variations (CV) and calculated as: (SD/mean)*100, where SD is the standard deviation of the measured variable (eg, Rrs6) and mean is the mean of all technically acceptable measured variables. Statistics were performed using SPSS for Windows, Version 12.0.1. Significance was accepted at the 0.05 level.
Cross‐sectional study
Relationships between respiratory function variables and anthropometric data (age, height, weight and gender) were examined. Stepwise and multiple regression analysis indicated that, after taking height into account, age, gender and weight did not significantly improve the prediction of respiratory function. The Fdep of Rrs between 4 and 24 Hz was independent of anthropometric data and these data were not included in prediction model analysis. Relationships between height and respiratory function investigated included linear, quadratic and power functions as these relationships have previously been reported in the studies of oscillatory mechanics in healthy children (as reviewed in1). We found that a linear relationship provided the best prediction of oscillatory mechanics and height in this group of healthy young children. Equations for the calculation of standardised SD or Z scores are reported (Z = (measured – predicted)/SEE), where “measured” is the measured respiratory function variable (eg, Rrs6), “predicted” is the predicted respiratory function variable determined from the regression equations and SEE is the standard error of the estimate of the regression equation.
The influence of a personal history of atopy and environmental tobacco exposure or a parental history of atopy or asthma on respiratory oscillatory mechanics was examined by including this information in the regression analysis of respiratory function and height. Personal atopy was examined as a binary variable, where a positive response was a history of eczema or hay fever ever or a positive skin prick test to any allergen. Children were considered to have experienced environmental tobacco exposure if either parent smoked. Parental history of asthma or atopy was defined as a reported history in either the mother or father of asthma, eczema or hay fever.
Short‐term repeatability studies
Between‐test repeatability was determined using the approach of Bland and Altman.16 Coefficients of repeatability (CR) are expressed in absolute (CR‐A) or relative to baseline as percentage (CR‐R) terms. The absolute coefficients of repeatability are calculated as 2*SD of differences calculated from the Bland and Altman analysis. The relative coefficients of repeatability are calculated as 2*SD of the differences between measurement sets as a percentage of the mean of the two measurements. The calculation of CR‐R allows a significant percentage change from baseline to be defined. The influence of respiratory history as a binary variable on the differences between measurements (both in absolute and relative terms) was compared using independent sample t tests.
Results
Cross‐sectional study
Respiratory impedance spectra were obtained in 158 healthy children aged between 26 months and 7 years with a height range of 92–127 cm. Anthropometric data are summarised in table 1. Poor coherence values (<0.95) at 6 Hz resulted in Rrs6 and Xrs6 data being available for fewer children (n = 149; 94%) at this frequency.
Within‐test variation of Rrs and Xrs data at 6–10 Hz are shown in table 2. Thirty‐three of the children had their data collected as part of a field study in which five successive measurements were collected without the child disconnecting from the equipment; all other children disconnected as desired. Children remaining on the equipment for all measurements had a significantly higher CV than those children allowed to rest between measurements. Median (10–90th centiles) CVs for Rrs at 6, 8 and 10 Hz in the field study were 11.2 (5.0–19.5)%, 9.5 (6.6–15.2)% and 9.4 (4.4–14.2)%, respectively, compared with 7.7 (3.4–15.5)%, 7.6 (2.7–12.6)% and 5.8 (3.6–12.0)% for Rrs at 6, 8 and 10 Hz, respectively, in laboratory‐based studies (p<0.05, Mann‐Whitney t test). Similarly, CVs for Xrs in the field study (32.2 (12.1–67.9)%, 25.4 (9.9–62.2)% and 19.4 (10.6–38.7)% for 6, 8 and 10 Hz, respectively) were more variable than laboratory‐based studies (17.6 (6.3–35.3)%, 17.9 (9.7–31.3)% and 15.8 (7.8–28.5)% for 6, 8 and 10 Hz, respectively; p<0.05, Mann‐Whitney t test).
Table 2 Within‐test variations and between‐test repeatability of respiratory impedance in young children.
| Within‐test variation | Short‐term repeatability (n = 58) | ||||
|---|---|---|---|---|---|
| SD (hPa.s/l) | CV (%) | N | Absolute (hPa.s/l) | Relative (%) | |
| Rrs6 | 0.68 (0.29–1.34) | 8.3 (4.0–16.8) | 149 | 2.04 | 32.06 |
| Rrs8 | 0.58 (0.23–1.08) | 8.0 (3.7–13.5) | 158 | 2.00 | 30.52 |
| Rrs10 | 0.47 (0.24–0.93) | 6.2 (3.7–12.6) | 158 | 1.76 | 28.14 |
| Xrs6 | 0.51 (0.21–1.04) | 20.5 (8.0–46.1) | 149 | 1.70 | 132.72 |
| Xrs8 | 0.44 (0.23–0.79) | 18.5 (9.8–34.4) | 158 | 1.22 | 94.78 |
| Xrs10 | 0.43 (0.18–0.77) | 16.4 (8.4–29.1) | 158 | 1.40 | 68.88 |
Rrs6, Rrs8, Rrs10, respiratory resistance at 6, 8 and 10 Hz; Xrs6, Xrs8, Xrs10, respiratory reactance at 6, 8 and 10 Hz.
Within‐test variations determined from the average of all acceptable measurements for an individual child. Data are expressed as absolute and relative within‐test variations. Absolute variations are reported as the median (10–90th centiles) of the standard deviation (SD) of respective forced oscillation technique (FOT) variables. Relative within‐test variation is reported as the coefficient of variation (CV) and is reported as median (10–90th centiles).
Short‐term repeatability data are reported in absolute and relative terms and are expressed as the coefficient of repeatability (CR). The CRs are calculated as 2*SD of the differences between measurements and are expressed in absolute (CR‐A, hPa.s/l) or relative (CR‐R, %) terms. See Methods for further details.
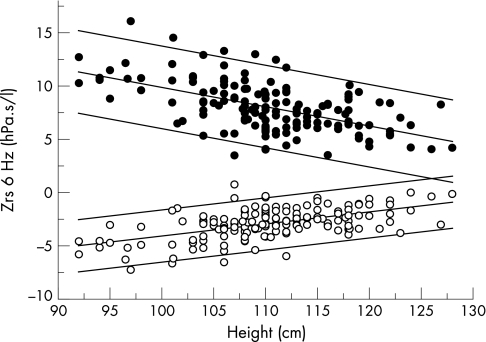
Figure 1 illustrates the relationship between body height and Rrs6 and Xrs6. The prediction equations for Rrs and Xrs are shown in table 3. The Fdep of resistance between 4 and 24 Hz was not related to children's body size, age or gender, so regression equations were not calculated for Fdep. Mean (SD) Fdep was −0.102 (0.075) hPa.s2/l , and thus the upper and lower limits (mean±2SD) of Fdep in this group of healthy children were −0.252 and 0.048 hPa.s2.l, respectively. Parental history of asthma, hay fever or eczema, or the child's personal history of atopy (eczema, hay fever or positive skin prick test) or environmental tobacco exposure did not significantly influence any of the respiratory function variables examined, after taking height into account.
Figure 1 Respiratory input impedance at 6 Hz against height in 149 healthy young children plotted as individual measurements of mean respiratory resistance at 6 Hz (Rrs6, closed circles) and respiratory reactance at 6 Hz (Xrs6, open circles). Predicted and 95% confidence intervals of Rrs6 and Xrs6 are plotted as solid lines.
Table 3 Regression data for respiratory impedance in healthy young children.
| Parameter | Equation | SEE | R2 | p Value |
|---|---|---|---|---|
| Rrs6 | 27.860 − (0.180 * Ht) | 1.918 | 0.311 | <0.001 |
| Rrs8 | 26.136 − (0.167 * Ht) | 1.754 | 0.309 | <0.001 |
| Rrs10 | 23.647 − (0.147 * Ht) | 1.567 | 0.304 | <0.001 |
| Rrs4–24 | 23.492 − (0.149 * Ht) | 1.543 | 0.317 | <0.001 |
| Xrs6 | −15.345 + (0.113 * Ht) | 1.212 | 0.306 | <0.001 |
| Xrs8 | −10.746 + (0.074 * Ht) | 1.024 | 0.207 | <0.001 |
| Xrs10 | −9.716 + (0.063 * Ht) | 1.069 | 0.148 | <0.001 |
Rrs6, Rrs8, Rrs10, respiratory resistance at 6, 8 and 10 Hz; Xrs6, Xrs8, Xrs10, respiratory reactance at 6, 8 and 10 Hz; Rrs4–24, average Rrs between 4 and 24 Hz.
Respiratory impedance data are expressed in hPa.s/l and Ht in cm.
SEE is the standard error of the estimate.
Short‐term repeatability studies
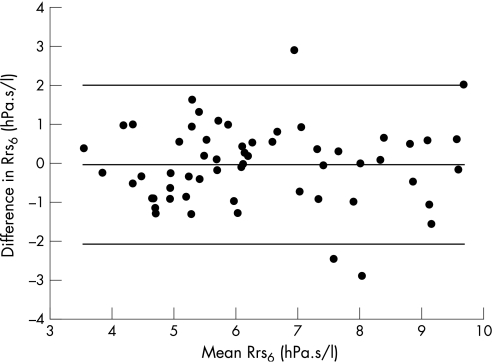
Paired data over a 15 min period were attempted in 69 children and were successful in 58, representing a success rate of 84% in children aged four to seven years. Differences between successive measurements (set 1 – set 2) were not related to the magnitude of the mean of the two measurement sets, height of the child or time between measurements (Pearson product moment correlation; p>0.05) when expressed in either absolute or relative terms. The group mean of the differences between measurement sets was not significantly different from zero and ranged from −0.17 to 0.14 h.Pa.s/l. These differences equated to a mean relative difference (difference as a percentage of the mean of the two measurements) of less than 2% for all FOT variables except for Xrs6 which had a relative mean difference of 3.8%. The coefficients of repeatability (2*SD of the differences) between measurement sets for absolute and relative relationships are shown in table 2. Figure 2 illustrates the Bland‐Altman analysis for Rrs6. Short‐term repeatability was not influenced by respiratory history (data not shown; p>0.05, Mann‐Whitney t test).
Figure 2 Bland‐Altman plot of the agreement in respiratory resistance at 6 Hz (Rrs6) between two measurement sets 15 min apart. Data are plotted as the difference between measurements 1 and 2 versus the mean of the measurement sets. Differences for individual children are shown as solid circles. The mean of the differences (−0.04 hPa.s/l) and the upper (2.00 hPa.s/l) and lower (−2.08 hPa.s/l) limits of agreement are plotted as solid lines.
Discussion
This study reports the relationships between height and various forced oscillatory variables in a cross‐sectional study of 158 healthy children aged between two and seven years of age using a pseudo‐random forcing signal. Information on the short‐term repeatability of these oscillatory variables in absolute and relative terms is also presented. These data provide a contemporary reference data set of forced oscillatory variables obtained using a new commercial FOT device in healthy pre‐school children.
Reference ranges for Zrs in young children
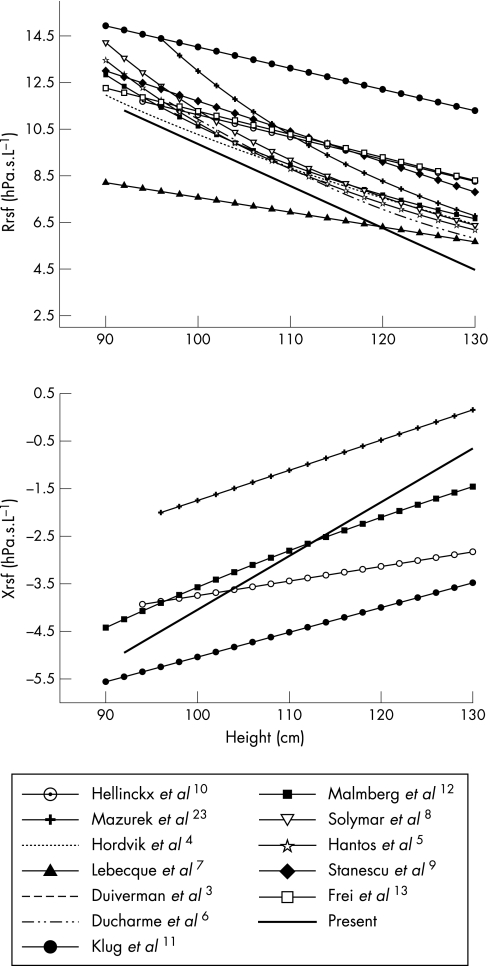
The clinical application of a respiratory function technique requires accurate reference data. In particular, careful attention must be paid to the anthropometric characteristics of the reference population, the equipment specifications and methodology used. Numerous reference ranges for forced oscillatory mechanics have been published, as reviewed by Oostveen et al.1 A comparison of the reference ranges from the present study and those previously published are shown in fig 3. The majority of existing studies, in particular those using pseudo‐random oscillatory signals, have reported Rrs alone and therefore changes in Xrs with height are less well characterised. The variations in reported reference data are probably due to differences in equipment, particularly signal generation, methodology and cohort effects. Lack of technique standardisation up to this point may also have contributed to some of this variation. In agreement with the current study, the majority of published FOT reference data have described an inverse relationship between Rrs and height, with no significant differences between genders. The study of Klug and Bisgaard11 collected FOT measurements using a facemask arrangement which may have contributed to the higher Rrs and more negative Xrs values for healthy children observed in this study.
Figure 3 Respiratory resistance (top panel) and reactance (bottom panel) data compared with existing studies of respiratory system impedance in healthy young children.
Repeatability of Zrs in young children
A description of the variability of oscillatory variables is required in order to define clinically relevant changes. In this study we report within‐test variability and between‐test repeatability of a range of Zrs variables. We found that the FOT collection protocol influenced the coefficients of variation of FOT variables, while there was no influence of a history of wheeze on between‐test repeatability.
The mean within‐test variation of Rrs has been reported to be between 6% and 11%11,12,17,18,19 and is comparable to the within‐test variations of Rrs reported in the present study (5–7% in the laboratory setting and 9–11% in the field setting). The intra‐test variation of Xrs is not well documented, particularly in studies using pseudo‐random forcing signals. Klug and Bisgaard,17,19 using an impulse oscillation system, reported within‐test variation of Xrs of 16–17% which is similar to the Xrs variations of 16–17% reported in the present study under laboratory conditions while the within‐test variability of Xrs in the field study was significantly higher at 19–30%. The major difference between these collection protocols related to the training of subjects. In the field study we recorded a fixed number of five Zrs spectra without a period of training before data collection. In contrast, in the laboratory protocol children were allowed to acclimatise to the FOT equipment and up to five technically acceptable measurements were obtained. We therefore consider that the within‐test variations of Rrs and Xrs obtained from the field studies represent the upper limit of variations to be expected in young children.
We characterised the short‐term between‐test repeatability in absolute and relative terms to baseline values. Few studies have assessed between‐test repeatability of forced oscillatory mechanics in young children. Malmberg et al12 reported repeatability in 19 children following placebo inhalation, with the SD of the change being 0.39 and 0.46 hPa.s/l for Rrs5 and Xrs5 respectively. Klug and Bisgaard11 reported repeatability over a 15 min period in 120 children aged two to seven years with a larger variability in Rrs5 and Xrs5 of 1.3 and 1.0 hPa.s/l, respectively. The SD of the differences in Rrs6 and Xrs6 in the current study was 1.02 and 0.85 hPa.s/l, respectively, and is comparable to those previously reported. The relative short‐term repeatability for Xrs may not provide a true indication of variability resulting from proximity of Xrs to zero in some children. While we have shown the relative coefficients of repeatability for Xrs for illustrative purposes, we believe that absolute changes in Xrs should be used.
Technical aspects of the study
Strict quality control was used in the current study to ensure that reliable Zrs measures were obtained. Measurements from individuals were only accepted if there was no evidence of physical artefact as assessed from the timed flow and pressure traces. Collection of measurements during quiet tidal breathing in younger children can be difficult with success rates in children less than four years of age reported to be lower than those in older children.11,21 As the population reported in the current study was identified from a number of other research studies, we are unable to document age‐related feasibility and success rates. However, in the field study examining short‐term repeatability, paired data collection was successful in 83% of children aged between four and seven years, with a single measurement set successfully obtained in 91% of children.
Critical to valid measurement of Zrs is the assumption that the relationship between applied forcing signal and measured output is linear. The coherence function allows this relationship to be quantified, and it has been suggested that coherence values should be >0.95.15 In the present study, data at frequencies with coherence function values of <0.95 were excluded, with Zrs spectra with three or more frequencies with low coherence function excluded. As a result, data at 6 Hz were obtained in fewer children (n = 149) than at 8 and 10 Hz (n = 158), equating to a loss of data at 6 Hz of approximately 6%. It is feasible that children with airway obstruction and/or increased respiratory rates may have lower rates of technically acceptable data at 6 Hz due to reduced signal to noise ratios at that frequency. If so, this will influence the choice of frequencies used for clinically related studies and further work is required to address this issue. The shunt induced by the compliant upper airways has been reported to introduce significant errors in the estimation of the Zrs.22 These authors, however, demonstrated that firm support of the cheeks and lower jaw act to minimise the errors associated with this shunt. In the present study an operator supported the child's cheeks during measurements; this has the added benefit of providing detailed feedback to the equipment operator on small mouth movements, swallows and/or leaks, hence increasing the identification of artifactual measurements. We are therefore confident that the relationships of oscillatory mechanics and size as well as short and medium term repeatability in the group of young children described in this study reflect the underlying respiratory mechanics of this group.
In summary, we have reported on respiratory system resistance and reactance in 158 healthy young children aged between two and seven years. In addition, the repeatability of respiratory system impedance over a 15 min period is described. These data, obtained with commercially available equipment using pseudo‐random noise forcing signals, provide a comprehensive description of oscillatory mechanics in healthy preschool children.
Abbreviations
Fdep - frequency dependence
FOT - forced oscillation technique
Rrs - respiratory resistance
Xrs - respiratory reactance
Zrs - respiratory system input impedance
Footnotes
This study was funded by the National Health and Medical Research Council, Australia, Department of Education, Western Australia, Austrian National Fund and the Asthma Foundation WA. The study sponsors had no involvement in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Competing interests: None.
References
- 1.Oostveen E, MacLeod D, Lorino H.et al The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003221026–1041. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrino R, Viegi G, Brusasco V.et al Interpretative strategies for lung function tests. Eur Respir J 200526948–968. [DOI] [PubMed] [Google Scholar]
- 3.Duiverman E J, Clement J, van de Woestijne K P.et al Forced oscillation technique. Reference values for resistance and reactance over a frequency spectrum of 2–26 Hz in healthy children aged 2.3–12.5 years. Bull Eur Physiopathol Respir 198521171–178. [PubMed] [Google Scholar]
- 4.Hordvik N L, Konig P, Morris D A.et al Normal values for forced oscillatory respiratory resistance in children. Pediatr Pulmonol 19851145–148. [DOI] [PubMed] [Google Scholar]
- 5.Hantos Z, Daroczy B, Gyurkovits K. Total respiratory impedance in healthy children. Pediatr Pulmonol 1985191–98. [DOI] [PubMed] [Google Scholar]
- 6.Ducharme F M, Davis G M, Ducharme G R. Pediatric reference values for respiratory resistance measured by forced oscillation. Chest 19981131322–1328. [DOI] [PubMed] [Google Scholar]
- 7.Lebecque P, Desmond K, Swartebroeckx Y.et al Measurement of respiratory system resistance by forced oscillation in normal children: a comparison with spirometric values. Pediatr Pulmonol 199110117–122. [DOI] [PubMed] [Google Scholar]
- 8.Solymar L, Aronsson P H, Bake B.et al Respiratory resistance and impedance magnitude in healthy children aged 2–18 years. Pediatr Pulmonol 19851134–140. [DOI] [PubMed] [Google Scholar]
- 9.Stanescu D, Moavero N E, Veriter C.et al Frequency dependence of respiratory resistance in healthy children. J Appl Physiol 197947268–272. [DOI] [PubMed] [Google Scholar]
- 10.Hellinckx J, De Boeck K, Bande‐Knops J.et al Bronchodilator response in 3–6.5 years old healthy and stable asthmatic children. Eur Respir J 199812438–443. [DOI] [PubMed] [Google Scholar]
- 11.Klug B, Bisgaard H. Specific airway resistance, interrupter resistance, and respiratory impedance in healthy children aged 2–7 years. Pediatr Pulmonol 199825322–331. [DOI] [PubMed] [Google Scholar]
- 12.Malmberg L P, Pelkonen A, Poussa T.et al Determinants of respiratory system input impedance and bronchodilator response in healthy Finnish preschool children. Clin Physiol Funct Imaging 20022264–71. [PubMed] [Google Scholar]
- 13.Frei J, Jutla J, Kramer G.et al Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest 20051281266–1273. [DOI] [PubMed] [Google Scholar]
- 14.Landser F J, Nagles J, Demedts M.et al A new method to determine frequency characteristics of the respiratory system. J Appl Physiol 197641101–106. [DOI] [PubMed] [Google Scholar]
- 15.Frey U. Forced oscillation technique in infants and young children. Paediatr Respir Rev 20056246–254. [DOI] [PubMed] [Google Scholar]
- 16.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 17.Klug B, Bisgaard H. Measurement of lung function in awake 2–4‐year‐old asthmatic children during methacholine challenge and acute asthma: a comparison of the impulse oscillation technique, the interrupter technique, and transcutaneous measurement of oxygen versus whole‐body plethysmography. Pediatr Pulmonol 199621290–300. [DOI] [PubMed] [Google Scholar]
- 18.Ducharme F M, Davis G M. Respiratory resistance in the emergency department: a reproducible and responsive measure of asthma severity (see comment). Chest 19981131566–1572. [DOI] [PubMed] [Google Scholar]
- 19.Bisgaard H, Klug B. Lung function measurement in awake young children. Eur Respir J 199582067–2075. [DOI] [PubMed] [Google Scholar]
- 20.Beelen R M, Smit H A, van Strien R T.et al Short and long term variability of the interrupter technique under field and standardised conditions in 3–6 year old children. Thorax 200358761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducharme F M, Davis G M. Measurement of respiratory resistance in the emergency department: feasibility in young children with acute asthma. Chest 19971111519–1525. [DOI] [PubMed] [Google Scholar]
- 22.Marchal F, Mazurek H, Habib M.et al Input respiratory impedance to estimate airway hyperreactivity in children: standard method versus head generator. Eur Respir J 19947601–607. [DOI] [PubMed] [Google Scholar]
- 23.Mazurek H, Willim G, Marchal F.et al Input respiratory impedance measured by head generator in preschool children. Pediatr Pulmonol 2000347–55. [DOI] [PubMed] [Google Scholar]