Abstract
Background
Despite national disease management plans, optimal asthma management remains a challenge in Australia. Community pharmacists are ideally placed to implement new strategies that aim to ensure asthma care meets current standards of best practice. The impact of the Pharmacy Asthma Care Program (PACP) on asthma control was assessed using a multi‐site randomised intervention versus control repeated measures study design.
Methods
Fifty Australian pharmacies were randomised into two groups: intervention pharmacies implemented the PACP (an ongoing cycle of assessment, goal setting, monitoring and review) to 191 patients over 6 months, while control pharmacies gave their usual care to 205 control patients. Both groups administered questionnaires and conducted spirometric testing at baseline and 6 months later. The main outcome measure was asthma severity/control status.
Results
186 of 205 control patients (91%) and 165 of 191 intervention patients (86%) completed the study. The intervention resulted in improved asthma control: patients receiving the intervention were 2.7 times more likely to improve from “severe” to “not severe” than control patients (OR 2.68, 95% CI 1.64 to 4.37; p<0.001). The intervention also resulted in improved adherence to preventer medication (OR 1.89, 95% CI 1.08 to 3.30; p = 0.03), decreased mean daily dose of reliever medication (difference −149.11 μg, 95% CI −283.87 to −14.36; p = 0.03), a shift in medication profile from reliever only to a combination of preventer, reliever with or without long‐acting β agonist (OR 3.80, 95% CI 1.40 to 10.32; p = 0.01) and improved scores on risk of non‐adherence (difference −0.44, 95% CI −0.69 to −0.18; p = 0.04), quality of life (difference −0.23, 95% CI −0.46 to 0.00; p = 0.05), asthma knowledge (difference 1.18, 95% CI 0.73 to 1.63; p<0.01) and perceived control of asthma questionnaires (difference −1.39, 95% CI −2.44 to −0.35; p<0.01). No significant change in spirometric measures occurred in either group.
Conclusions
A pharmacist‐delivered asthma care programme based on national guidelines improves asthma control. The sustainability and implementation of the programme within the healthcare system remains to be investigated.
The global prevalence of asthma ranges from 1% to 18% of the population1 and it is considered to be one of the most common chronic diseases worldwide.2 In Australia asthma affects 11% of adults and 14% of children,3 and is the most commonly reported long‐term condition in children aged 0–14 years.4 The economic burden of asthma is substantial and includes both direct (eg, hospital admissions and costs of medications) and indirect costs (eg, days away from work).2,5
Many countries now have national disease management plans for asthma,6,7,8,9,10 with the goals of reducing asthma severity and improving control. However, several studies have indicated less than optimal uptake of these plans.11,12,13 In Australia, the Six Step Asthma Management Plan has been accepted as the national guideline for the optimal management of asthma.14,15 Since 1990, the National Asthma Council (NAC) of Australia has promoted this plan to health professionals,15 undertaken epidemiological surveys on asthma, developed policies on asthma issues and conducted national public education campaigns.16 Despite this, asthma management practices in Australia remain suboptimal.17,18
It has been suggested that new strategies are needed within primary care to ensure that asthma care meets current standards of best practice.19 Community pharmacies offer accessible and convenient venues for patients with chronic illness and are often the first point of contact for people with asthma. Several pharmacy‐based asthma care models have been implemented to service people with asthma, and a variety of positive outcomes have been demonstrated. These include improved peak flow readings20,21,22 and symptoms scores,21,22,23 optimised drug use,20,21,22,24 reduction in healthcare utilisation20,25 and improvements in humanistic outcomes such as self‐efficacy and asthma knowledge.20,24 However, to our know ledge, none of the above mentioned service models adopted a standard set of national or international guidelines for the management of asthma.
In Australia a community pharmacy‐based asthma care model based on national guidelines (the NAC Six Step Asthma Management Plan) has been developed in consultation with pharmacists and was tested in a small pilot study.26 This model has subsequently been refined to become the Pharmacy Asthma Care Program (PACP). The objective of the current study was to implement the PACP in three Australian states (New South Wales, Queensland and Victoria) and evaluate its effect on asthma control and other clinical and humanistic patient outcomes.
Methods
Study design
The study used a multi‐site randomised intervention versus control repeated measures design. Based on detecting a 23% change in asthma severity26 with 90% power at the 5% significance level, and allowing for a 25% dropout rate and a cluster effect, a sample size of 195 in each group was determined. It was anticipated that a minimum of 40 pharmacies would be needed, recruiting an estimated 10 patients per pharmacy.
Approval for the study was given by the human research ethics committees of the University of Sydney, Monash University, Charles Sturt University and the University of Queensland. Written informed consent was obtained from all participating pharmacists and subjects. The study was initiated in November 2004 and completed in July 2005.
Pharmacists and patients
The sampling frame was all Quality Care Pharmacy Program (QCPP) accredited pharmacies (www.qcpp.com) in New South Wales, Victoria and Queensland located within 300 km of any of the four participating institutions. To ensure that rural and urban pharmacies were represented, the Pharmacy Access/Remoteness Index of Australia (PhARIA; www.gisca.adelaide. edu.au/projects/pharia.html) was used to stratify pharmacies. Invitations to participate in the study were made to 174 pharmacies, 65% of which were selected by random number generation from PhARIA 1 (highly accessible) and 35% from PhARIA ⩾2 (accessible to very remote). The proportions of PhARIA 1 and ⩾2 reflect population distribution in Australia.
Inclusion criteria for pharmacies were: QCPP accreditation, availability of a computer system compatible with the spirometer software to be used in the study, ability to attend training sessions and a minimum of two pharmacists on duty at any one time. The exclusion criterion for pharmacies was current involvement in any other research project.
Pharmacists were offered remuneration (AU$200) for their participation on a per patient completed basis. Fifty‐seven pharmacies agreed to participate and were randomly allocated to the intervention or control group (28 control and 29 intervention). Pharmacists were not informed as to group allocation; both groups were informed that they were providing an asthma care service involving spirometry.
Pharmacies were asked to recruit up to 10 subjects from their customers. Inclusion criteria for the subjects were age 18–75 years, previous diagnosis of asthma and fulfilment of one or more of the following subcriteria from the revised Jones' Morbidity Index:27
Use of a reliever medication >3 times a week over the previous 4 weeks.
Waking at night or morning with cough/chest tightness on at least one occasion over the previous 4 weeks.
Time off work/study because of asthma over the previous 4 weeks.
Symptoms of asthma (cough, breathlessness, wheeze, etc) at least once a week over the previous 4 weeks.
No visit to a doctor for asthma within the last 6 months.
Subjects were excluded from the study if they had a terminal illness, were currently enrolled in another clinical trial, did not self‐administer their inhaler and/or did not speak English well enough to communicate with the pharmacist and complete the questionnaires independently.
Pharmacist training
Intervention pharmacists were given an asthma education manual and were trained on risk assessment, pathophysiology of asthma, asthma medications, the NAC six‐step asthma management plan, patient education, goal setting, adherence assessment, spirometry (by qualified respiratory scientists) and the PACP protocol during a 2‐day workshop delivered by the research team. Control pharmacists were trained on risk assessment, spirometry and the control protocol during a 1‐day workshop. All pharmacies were provided with EasyOne spirometers, spirettes and software. The EasyOne spirometer was selected as it meets all American Thoracic Society/European Respiratory Society recommendations for diagnostic spirometers28 and has recently been shown to maintain calibration with routine clinical use.29
Outcome measures
Both clinical and humanistic outcomes were used to evaluate the service (table 1). The primary outcome measure was change in overall asthma severity/control. This was assessed using a tool adapted from the NAC's asthma severity assessment table (www.nationalasthma.org.au).15 Asthma severity/control was assessed based on self‐reported frequency during the previous month of asthma‐related symptoms (such as cough, wheeze, shortness of breath); waking at night due to asthma; chest tightness on waking; and limitation in vigorous or moderate physical activities. Subjects who reported “never” for all parameters were classified as “mild”, those who reported 0–1 times per week for any parameter were classified as “moderate”, and those who reported >1 times per week for any parameter were classified as “severe”.
Table 1 Measures used to evaluate the PACP.
| Outcome | Measure | Data source |
|---|---|---|
| Clinical | Asthma severity/control | NAC asthma severity assessment table15 |
| Lung function (FEV1, FEV1/FVC) | Spirometry | |
| Medication profile | Dispensed medication history | |
| Daily dose of medications | Dispensed medication history | |
| Inhaler technique | Inhaler technique checklist | |
| Adherence | Brief medication questionnaire30 | |
| Action plan ownership | Self‐reported data | |
| Humanistic | Asthma‐related quality of life | Asthma‐related quality of life questionnaire31 |
| Perceived control of asthma | Perceived control of asthma questionnaire32 | |
| Asthma knowledge | Consumer asthma knowledge questionnaire33 |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NAC, National Asthma Council.
Inhaler technique was assessed using device‐specific checklists26 which were consistent with GINA guidelines.9 Subjects who demonstrated all steps in the checklist in the correct order were classified as having correct inhaler technique. If necessary, the pharmacists then demonstrated the correct technique. The risk of non‐adherence was assessed using the Brief Medication Questionnaire;30 in addition, subjects were classified as adherent to preventer medications if they took 80–120% of preventer medication prescribed.
Procedures
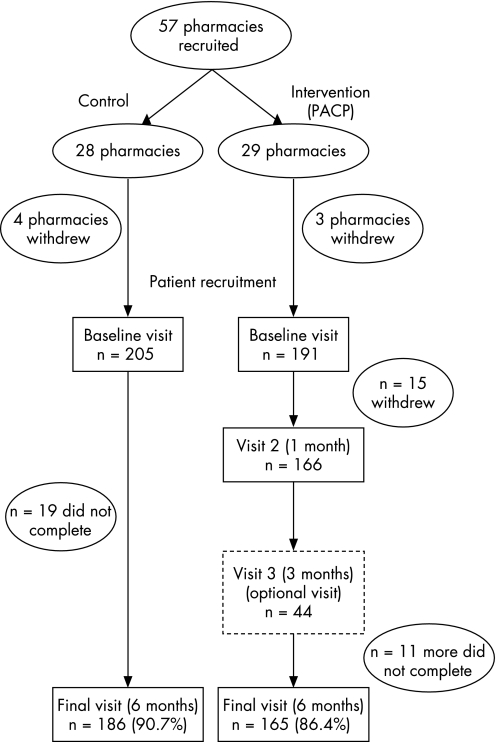
At the beginning of the study both intervention and control pharmacists assessed asthma severity/control,15 conducted lung function testing (by spirometry) and had patients complete questionnaires to collect baseline data on outcome measures (table 1). These were repeated 6 months later. Pharmacists were asked to achieve a spirometry quality grade of A, B or C in patient testing, with the lowest grade equivalent to at least two acceptable tests with the best two FEV1 values within 250 ml. Both groups also collected demographic details and asthma history. During the period between the baseline and 6 month visits, control patients received no intervention other than the pharmacist's usual care while the intervention pharmacies instituted the PACP, beginning at the baseline visit. The PACP (based on the NAC's Six Step Asthma Management Plan)14,15 provided an ongoing cycle of assessment, management and review in collaboration with general practitioners (GPs). The PACP included targeted counselling and education on the condition, medication and lifestyle issues (such as trigger factors); review of inhaler technique; adherence assessment; detection of drug‐related problems; goal setting and review; and referral to a GP as appropriate (eg, for a change in medication or dose). All interventions were documented manually. In addition to the baseline and 6 month visits, the intervention patients visited the pharmacy 1 month after the baseline visit and returned 3 months after the baseline visit if there were outstanding issues (fig 1).
Figure 1 Flowchart of recruitment to Pharmacy Asthma Care Plan (PACP) and completion.
Adherence to the study protocol was monitored and facilitated during visits of project staff to the pharmacies. Pharmacists were also contacted regularly by telephone and sent monthly newsletters to keep them informed and motivated. Separate newsletters were provided to control and intervention pharmacies.
Statistical analysis
All data were analysed using SPSS for Windows 10.0 or the SAS System for Windows 9.1. A two‐tailed significance level of 0.05 was used in all analyses. Between‐group differences at baseline were compared using independent sample Student's t test for continuous parameters and Pearson's χ2 test (with Yates' continuity correction in the case of dichotomous variables) for categorical parameters.
Outcomes for continuous parameters were evaluated using repeated measures multivariate ANOVA. For variables with differences at baseline, a univariate analysis of variance was conducted with baseline values as a covariate. For non‐normally distributed data, a non‐parametric test (Mann‐Whitney U test) was used to confirm the results of the parametric analysis. Outcomes of categorical parameters were evaluated by binomial logistic regression with baseline data and group as covariates. For categorical parameters conducted solely on the intervention group, McNemar's test was used to test for changes over time.
To account for any cluster effect (ie, correlation of patients within pharmacies),34 a multilevel logistic regression was performed on the primary outcome measure (asthma severity) using the SAS GLIMMIX procedure. Patients were defined as level 1 observations and pharmacies as level 2 observations.
As final visit data could not be collected on those who did not complete the study, outcome analyses were conducted on the available group only (ie, those who completed the study). However, to reduce any bias, the analyses of the primary outcome measure (asthma severity) was repeated using an intention‐to‐treat approach assuming that there was no change in initial values for those with missing final data.
Results
Comparison of control and intervention groups at baseline
During the course of the study, four control pharmacies and three intervention pharmacies failed to recruit any subjects, leaving 24 control and 26 intervention pharmacies. There were no significant differences in the characteristics of the participating pharmacies or pharmacists between the control and intervention groups (table 2).
Table 2 Characteristics of PACP pharmacies and pharmacists.
| Control | Intervention | |
|---|---|---|
| Number of participating pharmacies | 24 | 26 |
| Mean (SD) number of pharmacists on duty | 1.9 (0.7) | 2.0 (0.8) |
| Mean (SD) number of prescriptions dispensed per week | 1161 (617) | 1262 (810) |
| Location, N (%) | ||
| Stand‐alone | 18 (75) | 14 (56) |
| Shopping centre | 3 (12.5) | 4 (16) |
| Other | 3 (12.5) | 7 (28) |
| Number of participating pharmacists | 25 | 32 |
| Male/female (%) | 44/56 | 56/44 |
| Age group (years), N (%) | ||
| ⩽35 | 10 (40) | 14 (44) |
| 36–55 | 14 (56) | 12 (38) |
| ⩾56 | 1 (4) | 6 (19) |
| Owner/employee (%) | 52/48 | 44/56 |
A total of 396 patients were recruited and attended the baseline visit at their respective pharmacies, 205 in the control group and 191 in the intervention group. Of these, 186 control patients and 165 intervention patients completed the study (completion rates 90.7% and 86.4%, respectively). Forty‐five subjects (19 in the control group and 26 in the intervention group) did not complete the study (fig 1). Ten intervention patients did not complete either of the intermediate visits (visits 2 or 3) but did return for the final visit.
The baseline characteristics of the patients are summarised in table 3. There were no significant differences in the baseline characteristics between the patients who were recruited and those who completed the study. Patients in both the intervention and control groups had similar demographic characteristics, spirometric parameters, medication profiles, doses of asthma medications, adherence and humanistic measures at baseline (table 3). There was a higher proportion of previous smokers (p = 0.05) and patients with another lung disease in addition to asthma (such as chronic obstructive pulmonary disease) in the control group than in the intervention group (p<0.001). Patients in the control group also scored slightly better on the Brief Medication Questionnaire (p = 0.01). The asthma severity/control status of most of the patients (79%) was “severe”, with a higher proportion of patients with severe asthma in the intervention group than in the control group (88% vs 71%; p<0.001, table 3).
Table 3 Baseline characteristics of study patients.
| Control | Intervention | p Value* | |||
|---|---|---|---|---|---|
| Recruited | Completed | Recruited | Completed | ||
| Number of patients† | 205 | 186 | 191 | 165 | |
| Age at recruitment (years) | 50.4 (16.1) | 50.9 (15.9) | 47.5 (17.1) | 49.3 (17.0) | 0.08 |
| Male/female (%) | 39.5/60.5 | 39.2/60.8 | 32.5/67.5 | 30.3/69.7 | 0.18‡ |
| Asthma severity/control | |||||
| Mild | 3 (1.5%) | 3 (1.6%) | 5 (2.6%) | 5 (3.0%) | <0.001‡ |
| Moderate | 56 (27.7%) | 50 (27.2%) | 18 (9.4%) | 15 (9.1%) | |
| Severe | 143 (70.8%) | 131 (71.2%) | 168 (88.0%) | 145 (87.9%) | |
| Smoking status | |||||
| Current | 47 (23.0%) | 41 (22%) | 40 (20.9%) | 32 (19.4%) | 0.05‡ |
| Former | 45 (22.1%) | 40 (21.5%) | 26 (13.6%) | 22 (13.3%) | |
| Never | 112 (54.9%) | 105 (56.5%) | 125 (65.4%) | 111 (67.3%) | |
| Other lung disease | 39 (19.2%) | 37 (19.9%) | 15 (7.9%) | 14 (8.5%) | <0.001‡ |
| Spirometry | |||||
| FEV1 (% predicted) | 75.4 (22.5) | 74.1 (22.0) | 79.3 (22.8) | 79.7 (22.9) | 0.12 |
| FEV1/FVC (% predicted) | 86.2 (15.7) | 86.0 (15.6) | 87.8 (15.8) | 88.2 (15.9) | 0.37 |
| Daily dose of salbutamol (μg) | 382.8 (515.3) | 328.6 (371.9) | 436.3 (532.9) | 450.9 (566.2) | 0.41 |
| BMQ regimen screen¶ | 1.21 (1.11) | 1.17 (1.09) | 1.51 (1.09) | 1.51 (1.11) | 0.01 |
| AQLQ§ | 4.26 (1.53) | 4.21 (1.53) | 4.45 (1.40) | 4.45 (1.41) | 0.22 |
| CQ** | 7.68 (2.29) | 7.77 (2.24) | 7.80 (2.24) | 7.75 (2.23) | 0.59 |
| PCAQ†† | 25.09 (5.78) | 24.97 (5.71) | 26.15 (5.92) | 25.96 (5.85) | 0.08 |
| Correct inhaler technique | Not assessed | Not assessed | 41 (23%) | 39 (25.5%) | N/A |
| Asthma action plan | Not assessed | Not assessed | 36 (20.1%) | 36 (22.9%) | N/A |
| Adherent to preventer medication | 88 (57.9%) | 82 (60.3%) | 83 (53.2%) | 74 (55.2%) | 0.48‡ |
| Medication profile | |||||
| Reliever only | 31 (15.7%) | 30 (16.9%) | 24 (12.8%) | 22 (13.6%) | 0.51‡ |
| Preventer + reliever ± LABA | 166 (84.3%) | 148 (83.2%) | 163 (87.2%) | 140 (86.4%) | |
Values are mean (SD) or number (%).
FEV1, forced expiratory volume in 1 s; FEV1/FVC, forced expiratory volume in 1 second/forced vital capacity; BMQ, Brief Medication Questionnaire; AQLQ, Asthma‐related Quality of Life Questionnaire; CQ, Consumer Asthma Knowledge Questionnaire; PCAQ, Perceived Control of Asthma Questionnaire; reliever, short‐acting β2 agonists or anticholinergic bronchodilators; preventer, inhaled corticosteroids, theophylline derivatives, cromolyns, leucotriene receptor antagonists or oral corticosteroids; LABA, long‐acting β2 agonists.
*Comparing intervention and control groups at recruitment using independent samples t test unless otherwise noted.
†Not all patients responded to all questions and therefore numbers do not always add up to total.
‡χ2 test.
¶Range (best – worst): 1–7.
§Range (best – worst): 2–10.
**Range (worst – best): 0–12.
††Range (best – worst): 11–55.
Process evaluation
A total of 4747 interventions were delivered by the intervention pharmacists, with a mean (SD) of 27 (14) interventions per patient. The number of interventions delivered decreased with each subsequent visit. Overall, 96% (158/165) of intervention patients received interventions related to assessing asthma severity and control (such as identifying medication problems; education on asthma and its management; discussing health beliefs), 95% received medication adherence‐related interventions, and 93% received interventions related to identifying and avoiding trigger factors. Interventions related to not having or not using an action plan were delivered to 89% of intervention patients, and 76% received interventions related to their inhaler technique.
Intervention patients, in partnership with their pharmacist, set a mean (SD) of 3 (2) goals for themselves during the study. Fifty of 165 patients (30%) reported that they had achieved all of their goals by the end of the study, and 61% were continuing to work towards their goals. Eighty‐seven (53%) of the intervention patients set goals related to medications (eg, “remembering to take medication even when well” and “reducing Ventolin use”). Exercise tolerance (eg, “increase exercise” and “be more active”) was another common theme of goals (34% of intervention patients), as were asthma control (eg, “not wake up at night with asthma”) (34%) and lifestyle triggers (eg, “quit smoking” and “avoid triggers”) (27%).
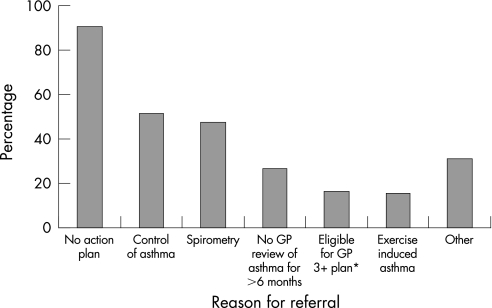
Eighty percent (131/164) of intervention patients were referred to their GP at least once during the study compared with only 21% (34/163) of the patients in the control group (p<0.001). The subsequent self‐reported referral uptake rate was 72% (94/131) for the intervention group and 47% (16/34) for the control group. The most common reason for referral was “Patient does not have a written asthma action plan” (fig 2).
Figure 2 Reasons for referral to GP of intervention patients (N = 128). Pharmacists could nominate one or more reasons per referral. *National Asthma Council of Australia.15
Primary outcome: asthma severity
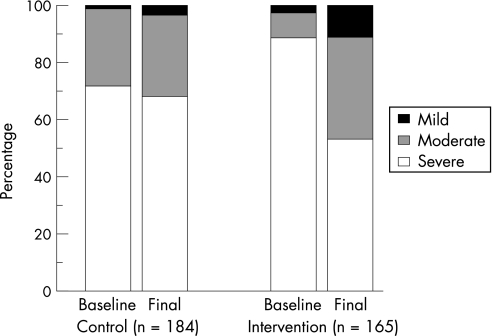
The proportion of intervention patients who were classified as having severe asthma declined significantly from 87.9% to 52.7% (p<0.001) during the study, while that of the control group remained unchanged (71.2% to 67.9%; p = 0.11; fig 3). A multilevel logistic regression model was used to adjust for the difference in severity at baseline and to account for any effect of cluster (ie, pharmacy), and found that patients in the intervention group were almost three times more likely to change from the “severe” category to the “not severe” category (“moderate” or “mild”) than patients in the control group (odds ratio (OR) 2.68, 95% CI 1.64 to 4.37; p<0.001). The intra‐pharmacy correlation coefficient (ie, cluster effect) was very small (–0.006). When a more conservative intention‐to‐treat approach was used, the results were similar (adjusted OR 2.42, 95% CI 1.51 to 3.88; p<0.001).
Figure 3 Asthma severity at baseline and at the 6 month (final) visit. The proportion of patients with severe asthma declined significantly in the intervention group but not in the control group (odds ratio 2.68, 95% CI 1.64 to 4.37; p<0.001).
Secondary outcome measures
There were no significant changes in spirometric parameters over the course of the study in either percentage predicted FEV1 or FEV1/FVC (table 4). Pharmacists achieved a quality grade of A, B or C in 85% of spirometric measurements; any measurements below grade C were excluded from the analysis.
Table 4 Secondary outcome measures.
| Outcome measure | Group | Mean change from baseline (95% CI) | p Value on change* | Mean difference (95% CI)† | p Value on difference‡ |
|---|---|---|---|---|---|
| Spirometry | |||||
| FEV1 (% predicted) | Intervention (n = 122) | −0.52 (−2.45 to 1.40) | 0.59 | −1.81 (−4.21 to 0.59) | 0.14 |
| Control (n = 135) | 1.29 (−0.20 to 2.78) | 0.09 | |||
| FEV1/FVC (% predicted) | Intervention (n = 122) | 1.39 (−0.29 to 3.08) | 0.10 | 0.41 (−1.76 to 2.57) | 0.71 |
| Control (n = 135) | 0.99 (−0.41 to 2.38) | 0.17 | |||
| Daily dose of salbutamol (μg) | Intervention (n = 90) | −145.4 (−263.4 to −27.3) | 0.02 | −149.1 (−283.9 to −14.4) | 0.03 |
| Control (n = 92) | 3.8 (−62.6 to 70.1) | 0.91 | |||
| BMQ regimen screen (range 1–7) | Intervention (n = 156) | −0.64 (−0.84 to −0.44) | <0.01 | −0.44 (−0.69 to −0.18) | 0.04 |
| Control (n = 173) | −0.20 (−0.36 to −0.04) | 0.01 | |||
| AQLQ (range 2–10) | Intervention (n = 160) | −0.64 (−0.83 to −0.45) | <0.001 | −0.23 (−0.46 to 0.00) | 0.05 |
| Control (n = 186) | −0.41 (−0.55 to −0.27) | <0.001 | |||
| CQ (range 0–12) | Intervention (n = 160) | 1.11 (0.80 to 1.41) | <0.001 | 1.18 (0.73 to 1.63) | <0.01 |
| Control (n = 184) | −0.07 (−0.40 to 0.25) | 0.67 | |||
| PCAQ (range 11–55) | Intervention (n = 153) | −2.53 (−3.36 to −1.70) | <0.001 | −1.39 (−2.44 to −0.35) | <0.01 |
| Control (n = 176) | −1.14 (−1.78 to −0.49) | 0.001 | |||
| Percent change from baseline (95% CI) | p Value on change§ | Odds ratio (95% CI) | p Value on odds ratio** | ||
| Correct inhaler technique | Intervention (n = 140) | 48.6% (39.2% to 58.0%) | <0.001 | NA | NA |
| Control | NA | NA | |||
| Asthma action plan | Intervention (n = 156) | 40.4% (31.9% to 48.9%) | <0.001 | NA | NA |
| Control (n = 191) | NA | NA | |||
| Adherence to preventer medication | Intervention (n = 127) | 16.6% (6.4% to 26.7%) | <0.01 | 1.89 (1.08 to 3.30) | 0.03 |
| Control (n = 121) | −1.7% (−11.9% to 8.6%) | 0.88 | |||
| Medication profile | |||||
| Intervention (n = 159) | Reliever only | –5.7% (−10.0% to −1.3%) | 0.02 | 3.80 (1.40 to 10.32) | 0.01 |
| Preventer + reliever ± LABA | 7.5% (1.8% to 9.5%) | ||||
| Control (n = 177) | Reliever only | 0% (−3.8% to 3.8%) | 1.00 | ||
| Preventer + reliever ± LABA | 0% (−3.8% to 3.8%) |
Values are mean (SD) or number (%).
NA, not applicable; FEV1, forced expiratory volume in 1 s; FEV1/FVC, forced expiratory volume in 1 s/forced vital capacity; BMQ, Brief Medication Questionnaire; AQLQ, Asthma‐related Quality of Life Questionnaire; CQ, Consumer Asthma Knowledge Questionnaire; PCAQ, Perceived Control of Asthma Questionnaire; reliever, short‐acting β2 agonists or anticholinergic bronchodilators; preventer, inhaled corticosteroids, theophylline derivatives, cromolyns, leucotriene receptor antagonists or oral corticosteroids; LABA, long‐acting β2 agonists.
*Paired t test unless otherwise noted.
†Change in intervention group – change in control group.
‡Repeated measures multivariate ANOVA unless otherwise noted.
¶ANCOVA.
§McNemar test.
**Binomial logistic regression.
When compared with the control group, the PACP intervention resulted in an increase in the proportion of patients adherent to preventer medications (OR 1.89, 95% CI 1.08 to 3.30), an improvement in the risk of non‐adherence to medications (indicated by a lower Brief Medication Questionnaire regimen score) (p = 0.04) and a decrease in the mean daily dose of the reliever medication salbutamol (p = 0.03). The intervention also resulted in an increase in the proportion of patients using a combination of reliever and preventer medications with or without a long‐acting β2 agonist (OR 3.80, 95% CI 1.40 to 10.32) as opposed to a reliever only. The proportion of intervention patients with correct inhaler technique increased significantly during the study (p<0.001), as did the proportion of patients with an asthma action plan (p<0.001, table 4). Inhaler technique and possession of an action plan were not measured in the control group.
Significant beneficial effects of the PACP intervention were seen in the Asthma Quality of Life score (p = 0.05), Consumer Asthma Knowledge scores (p<0.01) and Perceived Control of Asthma score (p<0.01, table 4).
Discussion
A national pharmacy‐based service (the PACP) for the care of patients with asthma resulted in improved clinical and humanistic outcomes. Over the 6 months of the study, interventions used by pharmacists resulted in improvements in asthma severity/control, adherence to preventer medication, quality of life, asthma knowledge and perceived control of asthma, as well as a decrease in the mean dose of the reliever medication salbutamol and in the number of patients relying solely on a reliever medication. Patients in the intervention group also had improved inhaler technique and more patients in this group had an action plan at 6 months compared with baseline.
These results are consistent with those achieved by other pharmacy‐based asthma care models.20,21,22,23,24 However, in this case, national asthma management guidelines were used as the framework for the service model so that current standards of best practice were met. In addition, the local needs of pharmacists were incorporated into the pilot model26 and therefore the sustainability of the service is likely to be higher.
The intervention pharmacists delivered a mean (SD) of 27 (14) interventions per patient. This suggests that management of asthma was less than optimal in the patients recruited to the study. In many previous studies, interviews and data collection have been performed by project staff and interventions carried out by the pharmacist.35 In this study the pharmacist was the driver of the service and documented everything he/she did. Most of the interventions focused on improving asthma control, adherence to medications, avoidance of trigger factors, inhaler technique and having an action plan. Inhaler technique was initially poor (24% with correct technique) and improved substantially by the end of 6 months (71% with correct technique). Adherence to preventer medications also improved (from 54% to 71%). Optimal inhaler technique will result in greater drug delivery and, in the case of preventer medication, lead to better control of inflammation and asthma. Further studies are needed to determine which of these interventions, alone or in combination, had a major impact on the improvement seen in asthma severity/control.
Ownership of an asthma action plan is a recommendation of the Six Step Asthma Management Plan.14 Action plans are intended to enable people with asthma to recognise deterioration promptly and respond appropriately, and thus aid patients in maintaining control of their condition.15 Referral for an action plan was the most commonly documented reason for GP referral. At baseline, the proportion of subjects with an action plan (23%) was similar to national data,18 and this improved significantly to 64% of intervention patients after 6 months. Nonetheless, it is still somewhat disappointing that not all patients had an action plan by the end of the study. While the reasons for this were not explored, we have previously reported that it is not possible to achieve ownership in 100% of people with asthma, despite referrals for this purpose.26 Perhaps in future a better collaborative interprofessional network would need to be established so that all healthcare professionals supported action plan ownership.
The goal setting process was an integral part of the PACP. Self‐management of a condition such as asthma is vital, given its chronic and episodic nature. Other studies have shown that patients retain and use self‐management skills effectively.23,36,37 By working with explicit goals that are personally relevant to the patient rather than set by the healthcare professional, the patient invests energy and enthusiasm, and behaviour change is more likely.
In most cases the PACP involved three visits over 6 months, and the follow‐up rate was extremely high, which suggests that patients found the service worthwhile. Whether this was also due to the motivation of the pharmacists was not tested. It is not known whether the service would need to be offered at the same intensity over a longer time period but, as the number of interventions decreased at each visit, the need for regular review should occur less often.
There are several limitations to this study. First, the patients recruited to the study were selected based on a risk assessment for poorly controlled asthma,27 so most of the patients were “severe” or “moderate” in terms of their asthma severity/control. Whether the positive results found in this study are generalisable to the whole community is not known. Second, there was a difference in asthma severity/control at baseline between the intervention and control group of patients. This concern was limited by performing a logistic regression with patients re‐categorised as “severe” or “not severe”. There were also more smokers and patients with other respiratory diseases in the control group. How this affected our results is uncertain. Third, the outcomes we described were achieved in 6 months; we do not know if they are sustainable long‐term or what further intervention would be required to sustain them. Fourth, there were some changes in the control group over time, suggesting that administration of the questionnaires may have changed pharmacist and/or patient behaviour. Finally, the diagnosis of asthma and the main outcome measure of asthma severity/control was based on self‐reported data.15 However, self‐reported asthma symptom scales are commonly used as an indicator of asthma severity20,21,22,23 and, in the pilot study for this project,26 improvements in peak flow indices were seen concurrently with improvements in asthma severity/control.
Pharmacists are underused healthcare professionals who frequently see patients with asthma in the community, many of whom may rarely consult a physician. Community pharmacies therefore represent an excellent site to screen for patients at risk from their asthma. This study has shown that they can also add value to the care of asthma, both in terms of clinical and humanistic outcomes for patients. Further studies are required to determine which components of the service are critical to improve asthma control and to determine the intensity of service required to sustain the improvement.
Acknowledgements
The authors thank the participating patients and community pharmacists. The reference group, Professor Sandra Anderson, Drs Ron Tomlins and Helen Reddel were all invaluable in the project design and implementation.
Abbreviations
FEV1 - forced expiratory volume in 1 s
FVC - forced vital capacity
NAC - National Asthma Council
PACP - Pharmacy Asthma Care Program
PhARIA - Pharmacy Access/Remoteness Index of Australia
QCPP - Quality Care Pharmacy Program
Footnotes
This work was funded by the Australian Department of Health and Ageing as part of the Third Community Pharmacy Agreement. The funding source had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of this paper; nor in the decision to submit this paper for publication.
Competing interests: None.
References
- 1.Masoli M, Fabian D, Holt S.et al The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 200459469–478. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Bousquet P J, Godard P.et al The public health implications of asthma. Bull WHO 200583548–554. [PMC free article] [PubMed] [Google Scholar]
- 3.Australian Bureau of Statistics National health survey: summary of results. Canberra: Australian Bureau of Statistics, 2001
- 4.Australian Institute of Health, Welfare (AIHW) Australia's health. Canberra: AIHW, 2004
- 5.Australian Centre for Asthma Monitoring Health care expenditure and the burden of disease due to asthma in Australia. Canberra: Australian Institute of Health and Welfare, 200543
- 6.North of England Asthma Guideline Development Group North of England evidence based guidelines development project: summary version of evidence based guideline for the primary care management in adults. BMJ 1996312762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulet L ‐ P, Becker A, Berube D.et al Canadian asthma consensus report, 1999. CMAJ 19991611S–25. [PMC free article] [PubMed] [Google Scholar]
- 8.Haahtela T, Klaukka T, Koskela K.et al Asthma programme in Finland: a community problem needs community solutions. Thorax 200156806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health Global Initiative for Asthma: global strategy for asthma management and prevention. Bethesda, Maryland: National Institutes of Health, 2002
- 10.National Asthma Education and Prevention Program Guidelines for the diagnosis and management of asthma. Expert Panel Report 2. Bethesda, Maryland: National Institutes of Health, National Heart, Lung, and Blood Institute, 1997
- 11.Adams R J, Fuhlbrigge A, Guilbert T.et al Inadequate use of asthma medication in the United States: results of the asthma in America national population survey. J Allergy Clin Immunol 200211058–64. [DOI] [PubMed] [Google Scholar]
- 12.de Marco R, Bugiani M, Cazzoletti L.et al The control of asthma in Italy. A multicentre descriptive study on young adults with doctor diagnosed current asthma. Allergy 200358221–228. [DOI] [PubMed] [Google Scholar]
- 13.Rabe K F, Adachi M, Lai C K W.et al Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol 200411440–47. [DOI] [PubMed] [Google Scholar]
- 14.Woolcock A, Rubinfeld A, Seale J.et al Thoracic society of Australia and New Zealand. Asthma management plan, 1989. Med J Aust 1989151650–653. [PubMed] [Google Scholar]
- 15.National Asthma Council Australia The asthma management handbook. Melbourne: National Asthma Council Australia, 200299
- 16.Comino E, Henry R. Changing approaches to asthma management in Australia: effects on asthma morbidity. Drugs 2001611289–1300. [DOI] [PubMed] [Google Scholar]
- 17.Matheson M, Wicking J, Raven J.et al Asthma management: how effective is it in the community? Intern Med J 200232451–456. [DOI] [PubMed] [Google Scholar]
- 18.Wilson D H, Adams R J, Appleton S L.et al Prevalence of asthma and asthma action plans in South Australia: population surveys from 1990 to 2001. Med J Aust 2003178483–485. [DOI] [PubMed] [Google Scholar]
- 19.Goeman D P, Aroni R A, Sawyer S M.et al Back for more: a qualitative study of emergency department reattendance for asthma. Med J Aust 2004180113–117. [DOI] [PubMed] [Google Scholar]
- 20.Cordina M, McElnay J C, Hughes C M. Assessment of a community pharmacy‐based program for patients with asthma. Pharmacotherapy 2001211196–1203. [DOI] [PubMed] [Google Scholar]
- 21.McLean W, Gillis J, Waller R. The BC Community Pharmacy Asthma Study: A study of clinical, economic and holistic outcomes influenced by an asthma care protocol provided by specially trained community pharmacists in British Columbia. Can Respir J 200310195–202. [DOI] [PubMed] [Google Scholar]
- 22.Narhi U, Airaksinen M, Tanskanen P.et al Therapeutic outcomes monitoring by community pharmacists for improving clinical outcomes in asthma. J Clin Pharm Ther 200025177–183. [DOI] [PubMed] [Google Scholar]
- 23.Barbanel D, Eldridge S, Griffiths C. Can a self‐management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 200358851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emmerton L, Shaw J, Kheir N. Asthma management by New Zealand pharmacists: a pharmaceutical care demonstration project. J Clin Pharm Ther 200328395–402. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Ohta M, Murata M.et al Decrease in emergency room or urgent care visits due to management of bronchial asthma inpatients and outpatients with pharmaceutical services. J Clin Pharm Ther 199823303–309. [DOI] [PubMed] [Google Scholar]
- 26.Saini B, Krass I, Armour C. Development, implementation, and evaluation of a community pharmacy‐based asthma care model. Ann Pharmacother 2004381954–1960. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama T, Chrystyn H. The Jones Morbidity Index as an aid for community pharmacists to identify poor asthma control during the dispensing process. Int J Pharmacy Pract 20031141–46. [Google Scholar]
- 28.Miller M R, Hankinson J, Brusasco V.et al Standardisation of spirometry. Eur Respir J 200526319–338. [DOI] [PubMed] [Google Scholar]
- 29.Walters J A E, Wood‐Baker R, Walls J.et al Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology 200611306–310. [DOI] [PubMed] [Google Scholar]
- 30.Svarstad B L, Chewning B A, Sleath B L.et al The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns 199937113–124. [DOI] [PubMed] [Google Scholar]
- 31.Marks G B, Dunn S M, Woolcock A J. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol 199245461–472. [DOI] [PubMed] [Google Scholar]
- 32.Katz P P, Yelin E H, Smith S.et al Perceived control of asthma: development and validation of a questionnaire. Am J Respir Crit Care Med 1997155577–582. [DOI] [PubMed] [Google Scholar]
- 33.Kritikos V, Krass I, Chan H S.et al The validity and reliability of two asthma knowledge questionnaires. J Asthma 200542795–801. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein H, Brown W J, Rasbash J. Multilevel modelling of medical data. Stat Med 2002213291–3315. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger M, Murray M D, Marrero D G.et al Effectiveness of pharmacist care for patients with reactive airways disease: a randomized controlled trial. JAMA 20022881594–1602. [DOI] [PubMed] [Google Scholar]
- 36.Caplin D L, Creer T L. A self‐management program for adult asthma. III. Maintenance and relapse of skills. J Asthma 200138343–356. [DOI] [PubMed] [Google Scholar]
- 37.Gibson P G, Powell H, Coughlan J.et al Self‐management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003(1)CD001117. [DOI] [PubMed]