Abstract
Background
Injurious mechanical ventilation can cause a pro‐inflammatory reaction in the lungs. Recent evidence suggests an association of the renin‐angiotensin system (RAS) with lung inflammation. A study was undertaken to investigate the pathogenic role of the RAS in ventilator‐induced lung injury (VILI) and to determine whether VILI can be attenuated by angiotensin converting enzyme (ACE) inhibition.
Methods
Male Sprague‐Dawley rats were mechanically ventilated for 4 h with low (7 ml/kg) or high (40 ml/kg) tidal volumes; non‐ventilated rats were used as controls. Lung injury and inflammation were measured by the lung injury score, protein leakage, myeloperoxidase activity, pro‐inflammatory cytokine levels and nuclear factor (NF)‐κB activity. Expression of the RAS components was also assessed. Some rats were pretreated with the ACE inhibitor captopril (10 mg/kg) for 3 days or received a concomitant infusion with losartan or PD123319 (type 1 or type 2 angiotensin II receptor antagonist) during mechanical ventilation to assess possible protective effects on VILI.
Results
In the high‐volume group (n = 6) the lung injury score, bronchoalveolar lavage fluid protein concentration, pro‐inflammatory cytokines and NF‐κB activities were significantly increased compared with controls (n = 6). Lung tissue angiotensin II levels and mRNA levels of angiotensinogen and type 1 and type 2 angiotensin II receptors were also significantly increased in the high‐volume group. Pretreatment with captopril or concomitant infusion with losartan or PD123319 in the high‐volume group attenuated the lung injury and inflammation (n = 6 for each group).
Conclusions
The RAS is involved in the pathogenesis of ventilator‐induced lung injury. ACE inhibitor or angiotensin receptor antagonists can attenuate VILI in this rat model.
Mechanical ventilation (MV) is indispensable in the management of critically ill patients with respiratory failure.1,2 However, MV can also subject the lungs to substantial abnormal stretching stress, resulting in structural changes, impaired gas exchange and activation of the inflammatory process leading to ventilator‐induced lung injury (VILI) and significant risk to the patients.3 Inflammatory cells and pro‐inflammatory mediators are considered to play an important role in the pathogenesis of VILI.4,5 Evidence supporting the inflammation‐inducing ability of injurious ventilation is also provided by the finding that mechanical stress can activate the inflammatory process via the nuclear factor (NF)‐κB pathway and this can be attenuated by glucocorticoids.6 However, the exact mechanism by which MV triggers the inflammatory process remains unclear.
Recently, the involvement of the renin‐angiotensin system (RAS) in the pathogenesis and evolution of inflammatory responses has received attention. On the basis of various experimental studies, the RAS is considered to be a key mediator of inflammation.7 Moreover, angiotensin II, the key factor of the RAS, has been shown in several in vitro studies to activate an inflammatory process by upregulation of the synthesis of pro‐inflammatory cytokines and chemokines via the type 1 (AT1) and type 2 (AT2) angiotensin II receptors and subsequent activation of the NF‐κB pathway.7,8,9 Systemic infusion of angiotensin II also activates NF‐κB in vivo in rat kidneys.10 On the other hand, the RAS is involved in cell death. Angiotensin II induces apoptosis of pulmonary endothelial cells11 and alveolar epithelial cells.12 The apoptosis can be abrogated by angiotensin receptor antagonists or other angiotensin II blockers.13 Moreover, angiotensin II also plays an important role in the fibrotic response to acute lung injury by inducing transforming growth factor‐α expression in the lungs.14 It is therefore likely that the RAS is highly involved in the development of lung injury with impaired pulmonary function. A recent paper supports the concept that angiotensin converting enzyme (ACE) aggravates the severe acute lung failure induced by acid aspiration and showed that mice deficient for the ACE gene have markedly reduced disease.15 It is therefore considered that, in conditions that can lead to lung inflammation such as high‐volume ventilation, the RAS may play an important role in the development of lung inflammation.
However, the role of the RAS in the activation of the inflammatory process associated with VILI and its molecular pathogenesis and the potential role of specific anti‐inflammatory therapeutic agents remain uncertain. We therefore investigated the involvement of the RAS in VILI. In this study we demonstrated that the RAS plays an important role in the development of VILI and that pretreatment with an ACE inhibitor or concomitant treatment with angiotensin receptor antagonists can attenuate the lung injury in this animal model.
Methods
Animal preparation, mechanical ventilation protocol and drug treatment
All experiments were performed after approval by the Institutional Animal Committee of the National Taiwan University College of Medicine.
Male Sprague‐Dawley rats weighing 200–250 g were anaesthetised by intraperitoneal injection of urethane (1.3 g/kg). Tracheostomy was performed and the tracheostomy tube was then connected to a volume‐controlled ventilator for small animals (New England Medical Instruments Inc, Medway, Massachusetts, USA). MV was applied using methods modified from the protocol described elsewhere.16,17 The animals were divided into the following three experimental groups: (1) non‐ventilated controls; (2) treated with MV with a high tidal volume (40 ml/kg tidal volume, 3 cm H2O of positive end‐expiratory pressure (PEEP), 20 breaths/min, room air); (3) treated with MV with a low tidal volume (7 ml/kg tidal volume, 3 cm H2O of PEEP, 100 breaths/min, room air). The control group only received the intraperitoneal injection of urethane. Mechanical ventilation was applied for 4 h and the peak airway pressure and mean arterial pressure were monitored throughout. Arterial blood gas analysis was performed using a portable analyser (i‐STAT, Abbott Laboratory, Abbott Park, Illinois, USA). After ventilation the animals were given a lethal dose of intraperitoneal pentobarbital then, after flushing the pulmonary vessels with intracardiac injection of normal saline, the lungs were removed en bloc. As a positive control for lung injury and inflammation, another group of non‐ventilated rats received E coli lipopolysaccharide instilled transtracheally (0.2 mg/100 g body weight dissolved in 0.5 ml phosphate buffered saline (PBS)).
Additional groups of rats received captopril pretreatment before MV or were treated with losartan or PD123319 during MV. For the captopril‐treated group, 50 mg/kg of captopril (Sigma Chemical, St Louis, Missouri, USA) was added to the drinking water (500 mg/l) for the 3 days preceding mechanical ventilation. In the losartan‐treated group, losartan (10 mg/kg) (a gift from Merck & Co Inc, Whitehouse Station, New Jersey, USA) was injected intravenously via a pump during the 4 h of MV; in the PD123319‐treated group, PD123319 (10 mg/kg; Sigma Chemical) was injected instead. All medicated animals underwent the same ventilatory strategies described above.
Histological studies
The left lungs (n = 6 for each group) were removed immediately after the animals were killed and fixed with a mixture of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacolate buffer, pH 7.4, for more than 24 h, dehydrated with a graded alcohol series and embedded in paraffin at 52°C. Sections were prepared and stained with haematoxylin and eosin for histological evaluation. Each lung section was scored for lung injury by a board‐certified pathologist using previously published criteria.18 Briefly, the scoring items included: (1) alveolar capillary congestion; (2) haemorrhage; (3) infiltration or aggregation of neutrophils in the air space or the vessel wall; and (4) thickness of the alveolar wall/hyaline membrane formation. Each item was graded according to the following five‐point scale: 0 = minimal (little) damage; 1 = mild damage; 2 = moderate damage; 3 = severe damage; and 4 = maximal damage. The mean sum of each field score was compared between the groups.
Myeloperoxidase assay
Myeloperoxidase (MPO) activity in the lung parenchyma, used as a marker enzyme for neutrophil infiltration into the lung, was assessed according to the methods described elsewhere.19,20
Bronchoalveolar lavage fluid protein measurement
Bronchoalveolar lavage (BAL) was performed as described previously.21 Total protein in the BAL fluid was determined using a commercial BCA Protein Assay Kit (Pierce, Rockford, Illinois, USA) according to the manufacturer's protocol as described previously.22
Tissue RNA extraction and reverse transcription and polymerase chain reaction (RT‐PCR)
Total RNA from the right lung of rats after 4 h after treatment was isolated using TRIzol reagent (Invitrogen, Carlsbad, California, USA). Tumour necrosis factor (TNF)α and macrophage inflammatory protein (MIP)‐2 mRNA levels in the lung tissue were examined by the reverse transcription and polymerase chain reaction (RT‐PCR), as described elsewhere.23 The gene‐specific oligonucleotide primers for TNFα, MIP‐2, and glyceraldehyde‐3‐phosphotate dehydrogenase (GAPDH) have been described previously.23
Real‐time reverse transcription‐polymerase chain reaction
Real‐time RT‐PCR to quantitatively measure mRNA levels for RAS components was performed in an ABI PRISM 7500 Sequence Detector (PE Applied Biosystems Inc).24 Oligonucleotide primers for rat angiotensinogen, ACE, angiotensin converting enzyme 2 (ACE2), and the AT1 and AT2 receptors were designed from the GenBank databases (NM 012544 and NM 134432; table 1) using Primer Express (PE Applied Biosystems Inc, Foster City, California, USA).
Table 1 Oligonucleotide used as primers for real‐time RT‐PCR.
| Gene name | Oligonucleotide | Sequence | Position | Amplicon |
|---|---|---|---|---|
| Agt | Forward primer | GTGGAGGTCCTCGTCTTCCA | 1239–1258 | 108 |
| Reverse primer | GTTGTAGGATCCCCGAATTTCC | 1346–1325 | ||
| Ace | Forward primer | CGGTTTTCATGAGGCTATTGGA | 3080–3101 | 102 |
| Reverse primer | TCGTAGCCACTGCCCTCACT | 3181–3162 | ||
| Ace2 | Forward primer | ACCCTTCTTACATCAGCCCTACTG | 755–778 | 74 |
| Reverse primer | TGTCCAAAACCTACCCCACATAT | 828–806 | ||
| Agtr1 | Forward primer | GAAGCCAGAGGACCATTTGG | 1260–1279 | 101 |
| Reverse primer | CACTGAGTGCTTTCTCTGCTTCA | 1360–1338 | ||
| Agtr2 | Forward primer | GCCAACATTTTATTTCCGAGATG | 528–550 | 81 |
| Reverse primer | TTCTCAGGTGGGAAAGCCATA | 608–588 |
Agt, angiotensinogen; Ace, angiotensin‐converting enzyme; Ace2, angiotensin‐ converting enzyme 2; Agtr1, type 1 angiotensin II receptor; Agtr2, type 2 angiotensin II receptor.
Measurement of lung tissue angiotensin II levels
An angiotensin EIA kit (Peninsula Laboratories, San Carlos, California, USA) was used to measure lung tissue angiotensin II levels according to the manufacturer's instructions, as described elsewhere.25
Western blot analysis for NF‐κB and I‐κB
Western blotting was used to assess the activation of NF‐κB in the lung tissue. Polyclonal antibodies against the p65 component of NF‐κB (Santa Cruz, Santa Cruz, California, USA), I‐κB or phosphorylated inhibitor‐κB (I‐κB) (both from Cell Signaling, Beverly, Massachusetts, USA) were used according to the methods described elsewhere. Antibodies to proliferating cell nuclear antigen (PCNA) or c‐Jun N‐terminal kinase 1 (JNK1) were used to detect these proteins, used as loading controls for the nuclear and cytosolic samples, respectively.
Western blot analysis for the RAS components and AT1 and AT2 receptors
Western blotting was used to assess the protein expression of the RAS components in the lung tissue. Polyclonal antibodies against ACE, ACE2, and AT1 and AT2 receptors (Santa Cruz) were used according to the methods described elsewhere.
Statistical analyses
All continuous data are expressed as mean (SD) values. Comparisons of continuous variables between groups were performed using the t test and one‐way analysis of variance using SPSS 10 software (SPSS Inc, Chicago, Illinois, USA). A p value of <0.05 was considered significant.
A detailed description of the Methods is available in the online supplement at http://thorax.bmj.com/supplemental.
Results
Physiological changes during MV
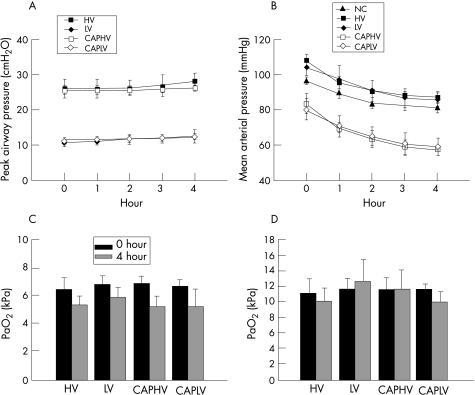
Peak airway pressure was higher in the high‐volume MV group than in the low‐volume MV group (fig 1A), but did not change significantly throughout the course of MV. The mean arterial pressure was markedly decreased during MV in rats pretreated with captopril (fig 1B) compared with the high‐volume and low‐volume MV groups. The arterial blood gas data were similar in MV groups at the beginning of MV and showed no significant change after 4 h of MV (fig 1C, D).
Figure 1 Change in (A) peak airway pressure, (B) mean arterial pressure, (C) arterial carbon dioxide tension (Paco2) and (D) arterial oxygen tension (Pao2) in different groups of animals (n = 6 for each group). The peak airway pressure was lower in the low‐volume ventilated groups than in the high‐volume ventilated groups (p<0.001). Pretreatment with captopril did not change the peak airway pressure. The mean arterial pressure was lower in the captopril‐pretreated animals (p<0.001 at the end of mechanical ventilation). Paco2 and Pao2 were similar between different groups and did not change significantly after 4 h of mechanical ventilation. HV, high‐volume ventilation; LV, low‐volume ventilation; CAPHV, captopril pretreatment followed by high‐volume ventilation; CAPLV, captopril pretreatment followed by low‐volume ventilation; NC, non‐ventilated control group.
Effect of high‐volume ventilation on lung injury and neutrophil infiltration in rat lungs and attenuation by captopril
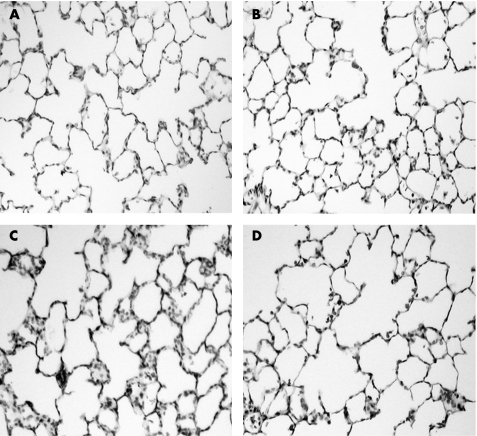
Histological studies showed that inflammatory cell infiltration was not significant in the lungs of the control group (fig 2A) or the low‐volume ventilation group (fig 2B), but high‐volume ventilation resulted in mild lung injury with mild neutrophil infiltration (fig 2C). The alveolar architecture was generally preserved. The neutrophil infiltration in the high‐volume group was attenuated by captopril pretreatment (fig 2D).
Figure 2 Haematoxylin and eosin staining of lung sections from (A) the non‐ventilated group, (B) the low‐volume ventilation group, (C) the high‐volume ventilation group and (D) the group pretreated with captopril followed by high‐volume ventilation. Original magnification ×200.
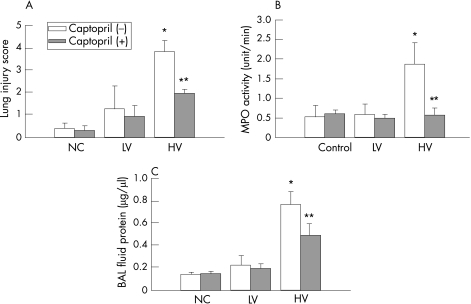
The pathological lung injury scores were compatible with the histological data, as high‐volume ventilation increased the lung injury score compared with the non‐ventilated controls, and the effect was significantly attenuated by captopril pretreatment (fig 3A). There was no significant difference between the scores for the low‐volume ventilation group and the controls or between the low‐volume ventilation groups with or without captopril pretreatment. Figure 3B shows that high‐volume MV significantly increased MPO activity and this effect was significantly attenuated by captopril pretreatment. There was no significant difference in MPO activity between the non‐ventilated control and the low‐volume ventilation groups. High‐volume ventilation also increases protein leakage into the alveolar space, and this was reduced by captopril pretreatment (p<0.05; fig 3C).
Figure 3 (A) Pathological lung injury scores for the non‐ventilated control, low‐volume ventilation (LV) and high‐volume ventilation (HV) groups (n = 6 for each group). The lung injury score in the HV groups was higher than that in the control group (**p<0.001) and was reduced by captopril (*p<0.001). The difference in lung injury score between the LV and control groups was not significant (p = 0.09). (B) Myeloperoxidase (MPO) activity was increased by HV (**p = 0.002) and was attenuated by captopril pretreatment (**p = 0.002, n = 3 for each group). (C) Bronchoalveolar lavage (BAL) fluid protein concentration was increased in the HV group (*p = 0.008) and was attenuated by captopril (*p = 0.027, n = 3 for each group).
Effect of captopril on lung expression of pro‐inflammatory cytokines and chemokines induced by high‐volume ventilation
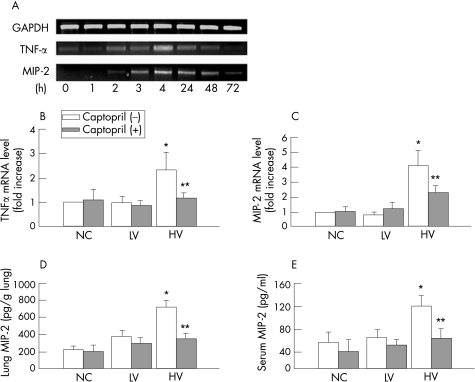
In the high‐volume ventilation group the mRNA levels of TNFα and MIP‐2 increased progressively up to 4 h, then decreased gradually after cessation of MV (fig 4A). The high‐volume ventilation group had significantly higher levels of TNFα and MIP‐2 mRNA than control groups; these increases were significantly attenuated by captopril pretreatment (fig 4B, C). The same trend was also shown for lung MIP‐2 and serum MIP‐2 protein levels by ELISA (fig 4D, E).
Figure 4 Effect of mechanical ventilation on the expression of pro‐inflammatory cytokines in rat lungs. (A) Time course of changes in lung mRNA levels of tumour necrosis factor (TNF)α and macrophage inflammatory protein (MIP)‐2 in a rat treated for 4 h with high‐volume (HV) ventilation. The levels of both peaked at 4 h. (B) and (C) Expressions of TNFα (p = 0.03) or MIP‐2 mRNA (p = 0.006) were increased in the lung in the HV group compared with the control group (n = 3 for each group; the increases were attenuated by captopril (**p = 0.045 for TNFα; **0.045 for MIP‐2). (D) The level of MIP‐2 in lung tissue was increased by HV ventilation (*p = 0.001) and was significantly reduced by captopril pretreatment (**p = 0.002; n = 3 for each group). (E) The serum level of MIP‐2 was significantly increased by HV (*p = 0.01) and was reduced by captopril pretreatment (**p = 0.02, n = 3 for each group).
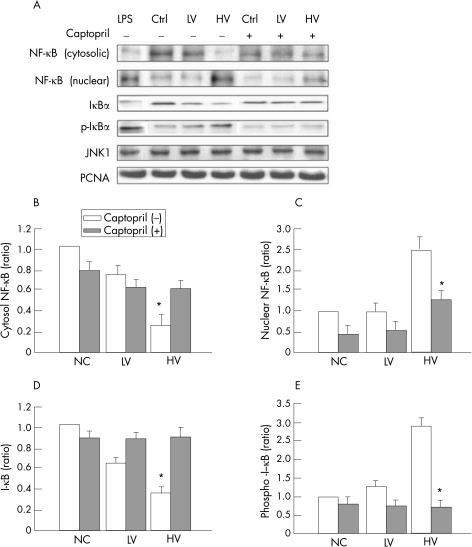
Effect of captopril on translocation of NF‐κB in rats with high‐volume ventilation‐induced lung inflammation
Figure 5 shows representative findings for NF‐κB activity after 4 h of treatment. The cytosolic fraction of NF‐κB was markedly decreased in the high‐volume ventilation and lipopolysaccharide‐treated groups, with a simultaneous distinct increase in nuclear NF‐κB (fig 5), showing that nuclear translocation of this factor was induced by injurious MV or lipopolysaccharide. In addition, translocation of NF‐κB in the high‐volume ventilation group was significantly, but not completely, attenuated by captopril pretreatment. On the cytosolic protein blots, the amount of I‐κB was also decreased by high‐volume MV, with a simultaneous increase in phosphorylated I‐κB. This increase in I‐κB phosphorylation was also attenuated by captopril pretreatment.
Figure 5 Effect of captopril on the activation of nuclear factor‐κB (NF‐κB) in rat lungs induced by mechanical ventilation. (A) Representative findings of Western blots after 4 h of treatment. (B–E) Relative levels of proteins measured by densitometry of the blots. High‐volume ventilation decreased the cytosol NF‐κB (p<0.001) and I‐κB (p<0.001) levels and increased the nuclear NF‐κB (p = 0.001) and cytosol phosphor‐I‐κB (p<0.001) levels. Captopril pretreatment increased the cytosol NF‐κB (p = 0.01) and I‐κB (p = 0.001) levels and decreased the nuclear NF‐κB (p = 0.005) as well as phosphor‐I‐κB (p<0.001) levels. Ctrl, non‐ventilated control group; JNK1, c‐Jun N‐terminal kinase 1; PCNA, proliferating cell nuclear antigen; NC, normal controls; HV, high‐volume ventilation; LV, low‐volume ventilation.
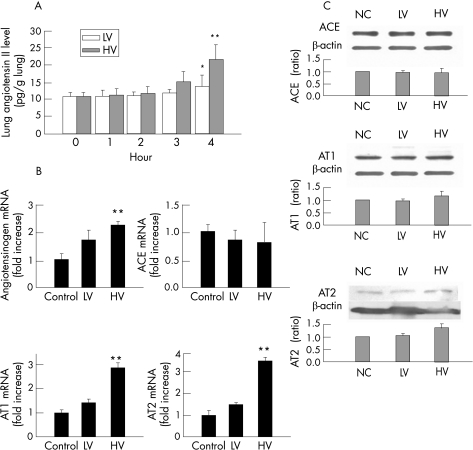
Effect of high‐volume mechanical ventilation on the expression of components of the RAS
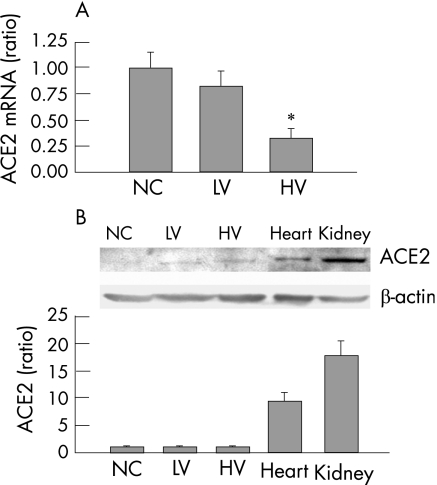
The lung tissue angiotensin level was progressively increased in the high‐volume group, but not in the low‐volume group, during the 4 h course of MV (fig 6A). Quantitative real‐time RT‐PCR of the lung tissue showed that high‐volume ventilation increased mRNA levels for angiotensinogen and the AT1 and AT2 receptors, but had no significant effect on ACE mRNA levels (fig 6A). The protein levels of the ACE, AT1 and AT2 were similar between groups. We also assessed the mRNA and protein levels of ACE2 in the lungs (fig 7). The mRNA expression of ACE2 was significantly decreased by high‐volume ventilation but not by low‐volume ventilation (fig 7A). The lung levels of ACE2 protein of the rats were very low compared with the level in the kidney or heart (fig 7B). The difference between groups was not significant.
Figure 6 Effect of mechanical ventilation on the expression of components of the rennin‐angiotensin system. (A) Enzyme immunoassay for lung tissue angiotensin II (n = 6 for each group). High‐volume (HV) ventilation significantly increased angiotensin II (**p = 0.007) whereas low‐volume (LV) ventilation did not (*p = 0.69). (B) Quantitative real‐time reverse transcription and polymerase chain reaction (RT‐PCR) for mRNA levels for angiotensinogen, angiotensin converting enzyme (ACE) and types 1 and 2 angiotensin II receptors (AT1 and AT2) (n = 3 for each group). Angiotensinogen (p = 0.01), AT1 (p<0.001) and AT2 (p = 0.008) mRNA were increased by HV whereas ACE mRNA was decreased (p = 0.002). (C) Western blotting for ACE, AT1 and AT2. The differences in lung tissue levels for ACE (p = 0.85) and AT1 (p = 0.089) were not significant between the control and HV groups, but the AT2 level was significantly increased in the HV group (**p = 0.002).
Figure 7 Expression of angiotensin converting enzyme 2 (ACE2) mRNA and protein in rat lung tissue. (A) Real‐time reverse transcription and polymerase chain reaction (RT‐PCR) shows that the mRNA expression of ACE2 was significantly decreased by high‐volume (HV) (p<0.001) but not by low‐volume (LV) (p = 0.067) ventilation (n = 6 for each group). (B) The lung levels of ACE2 protein of the rats were very low compared with the level in the kidney or heart (control group (NC) lung vs kidney, p = 0.007; lung vs heart, p = 0.017). The difference between groups was not significant. (HV vs NC, p = 0.90, n = 3 for each group).
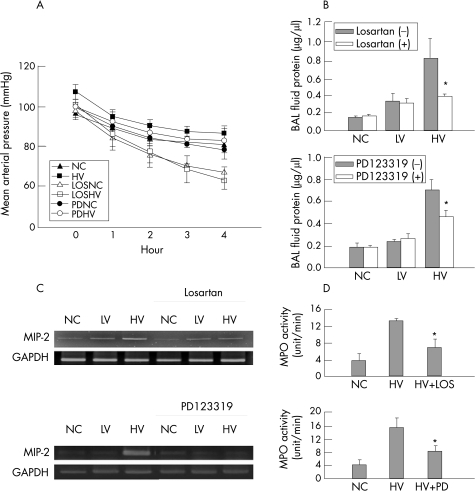
Effects of angiotensin II receptor blockade on high‐volume ventilation‐induced expression of pro‐inflammatory chemokine
During mechanical ventilation the decrease in blood pressure became more profound in the losartan‐treated group but not in the PD123319‐treated rats (fig 8A). Concomitant infusion of either losartan or PD123319 during MV attenuated the protein leak into the BAL fluid (fig 8B). The increase in lung tissue levels of MIP‐2 mRNA induced by high‐volume ventilation was attenuated by the concomitant infusion of either losartan or PD123319 (fig 8C). The increase in lung tissue myeloperoxidase activity by high‐volume ventilation was also attenuated by losartan and by PD123319 (fig 8D).
Figure 8 Effects of losartan and PD123319 on ventilator‐induced lung injury. (A) Mean arterial pressures were significantly decreased by losartan (high‐volume (HV) ventilation groups, p<0.001) but not by PD123319 (p = 0.23 between HV groups; n = 6 for each groups). (B) The increase in BAL fluid protein concentration by HV was significantly decreased by losartan (*p = 0.03) and by PD123319 (*p = 0.03; n = 3 for each group). (C) The increase in macrophage inflammatory protein (MIP)‐2 mRNA levels induced by HV was attenuated by losartan and by PD123319. The experiment was repeated three times with similar results. (D) Myeloperoxidase (MPO) assay shows a significant reduction in HV‐induced MPO activity by losartan (*p = 0.004) and by PD123319 (*p = 0.014). LOSHV, high‐volume ventilation with losartan infusion; LOSNC, non‐ventilated control with losartan infusion; PDNC, non‐ventilated control with PD123319 treatment; PDHV, high‐volume ventilation with PD123319 treatment; BAL, bronchoalveolar lavage.
Discussion
In this study we have reported two novel findings. First, the RAS is activated by high tidal volume MV in the in vivo animal model. Over‐distension of lung units by high‐volume ventilation resulted in upregulated expression of RAS components and the AT1 and AT2 receptors and increased lung angiotensin II production. Second, the RAS plays an important role in high‐volume VILI. Treatment with an ACE inhibitor or angiotensin receptor antagonist attenuated VILI, with suppression of cytokine expression and NF‐κB activity in the lungs. We therefore believe that the RAS is actively involved in the pathogenesis of VILI.
Although previous clinical and experimental studies have shown that a high tidal volume results in injury to the lung,2,6,26 the consequences of high‐volume ventilation in in vivo animal models, as seen in the current report, may be far more complex. Factors associated with the development of ventilator‐induced lung injury may include the species of animals used in the study, the compliance of the respiratory system and the tolerance of the animal to the MV protocol. One of the main features of our study design was that we used a very high (40 ml/kg) tidal volume to cause lung injury, as had been reported in the literature. In experimental studies, as the tidal volume is increased to exert a pathogenic effect, the respiratory rate is usually reduced to maintain the arterial blood gas data constant, as in the current study. In other studies additional carbon dioxide was added to the breathing air to compensate the hyperventilation‐induced hypocapnia in the high tidal volume group, while the respiratory rate was maintained. In our experience, we found that the rats could not tolerate a high respiratory rate due to severe haemodynamic compromise (data not shown). This finding was probably due to severe air trapping, which markedly increased the intrathoracic pressure and compromised venous return. We therefore cannot exclude a confounding effect of the respiratory rate on lung injury caused by MV. The protocol used in this study was based on those used by Whitehead et al16 and Ricard et al,17 in that a very high tidal volume (about 40 ml/kg) was used to create injurious inflation of the alveoli. The reason for using this extremely high tidal volume was that the rats have good compliance of the respiratory system, and ventilation with this large volume does not cause too great an increase in the plateau airway pressure. Another consideration is that this treatment may mimic some extreme clinical conditions such as the adult respiratory distress syndrome (ARDS), in which the number of remaining functional alveoli may be so low that ventilation with a more normal tidal volume is considered as a very large volume. We did not find overt histological evidence of alveolar disruption in the lungs of rats ventilated with 40 ml/kg tidal volume, so the finding of neutrophil infiltration in the lungs cannot be explained by stress‐related cell disruption or cell death. Activation of the lung cells, so‐called mechanotransduction, may instead play an important role in the development of lung injury in this model. Another point in our study design is that the minute volumes of the low‐ and high‐volume groups were different. The reason for the choice of these settings was that the arterial blood gas data were similar in the two groups during ventilation.
The RAS has been considered a mediator of inflammation7 and may therefore play an important pathogenic role in the inflammatory process associated with VILI. There have been previous reports that the pulmonary RAS and angiotensin II are associated with various pathophysiological conditions.27,28,29 Most reports supporting the active role of angiotensin in lung inflammation and injury involved studies on cultured cells.12,13 Imai et al15 recently showed, in an in vivo study, that ACE is an important regulator of acid‐induced lung injury. Our findings may provide further in vivo evidence for the involvement of the RAS in VILI. The pathogenic role of RAS in the inflammatory process and VILI is strongly supported by the fact that pretreatment with captopril attenuated lung inflammation, suppressed TNFα and MIP‐2 expression and NF‐κB activity and reduced blood levels of angiotensin II. Based on these findings, we believe that ACE inhibition may be beneficial in animals ventilated with injurious high tidal volumes.
Our findings also provide support for the proposal that NF‐κB pathway is the downstream response pathway for the action of angiotensin II30 in the inflammation process, as ACE inhibition attenuated the nuclear translocation of NF‐κB. However, the RAS may also be influenced by NF‐κB, as inhibition of NF‐κB also attenuates RAS activity.31 Moreover, NF‐κB is necessary for upregulation of AT1 receptor mRNA in rat cardiac fibroblasts treated with TNFα or interleukin‐1β.32 Together with our findings, these results suggest that a positive feedback system may be present, and this possibly explains why high‐volume MV triggers the RAS and why RAS‐mediated ventilator‐induced lung inflammation developed rapidly in our animal model study. In addition, angiotensin II has been shown to be associated with apoptosis and the fibrogenic process in lung injury,14 but whether these are associated with VILI requires further exploration.
Our finding that captopril treatment attenuated VILI and the inflammation process in the animal model may have important clinical implications. Therapeutic agents that block the production (ACE inhibitors) or action (angiotensin II receptor antagonist) of angiotensin II may be used as anti‐inflammatory agents for the treatment or prevention of VILI. The activation of NF‐κB associated with VILI is partially blocked by corticosteroids.6 Although there are few reports of the role of ACE inhibitor treatment in lung inflammatory diseases, a recent report showed that ACE inhibitor treatment is associated with a 19% lower risk of pneumonia, and the effect is more significant in Asian patients who have a 47% risk reduction.33 In our study, the lung inflammation action of angiotensin II could be mediated through both the AT1 and AT2 receptors. In the study by Imai et al,15 the AT1 receptor promoted lung disease whereas the AT2 receptor improved lung function. However, another study showed that NF‐κB can be inactivated by administration of ACE inhibitor or AT1/AT2 receptor blockade.34 In our animal model the dose of PD123319 was relatively high (42 μg/kg/min), so it is possible that the AT1 receptor might also be affected by PD123319.35 The role the AT2 receptor in the pathogenesis of VILI therefore requires further study. Our findings that AT2 receptor blockade could attenuate VILI may provide further information on the complex mechanisms for lung injury.
The main limitation of this study is that it focused on the short‐term effects of injurious MV and data regarding long‐term effects are lacking. However, we have shown that active inflammation in the lungs can develop early in injurious MV and we believe that, if these injurious settings were used for a longer period, the inflammation and injury would be further aggravated. Although the inflammation was attenuated by an ACE inhibitor or angiotensin receptor antagonists, we do not know the long‐term consequence of drug treatment, especially in the clinical setting in which treatment with these agents can cause hypotension, which is clearly undesirable in critically ill patients. Another limitation is that this study involved injurious MV as a pure insult to originally healthy lungs. This may be different from most clinical scenarios in which the patients may suffer from other initial insults such as pneumonia, sepsis or even ARDS before receiving MV. The local ACE and ACE2 activities were also not measured in this study. Further investigations are needed to elucidate the detailed mechanism of action of the RAS in VILI.
A detailed description of the Methods is available in the online supplement at http://thorax.bmj.com/supplemental
Supplementary Material
Abbreviations
ACE - angiotensin converting enzyme
ACE2 - angiotensin converting enzyme 2
AT1 - AT2, types 1 and 2 angiotensin II receptors
BAL - bronchoalveolar lavage
JNK1 - c‐Jun N‐terminal kinase 1
MIP‐2 - macrophage inflammatory protein
MPO - myeloperoxidase
MV - mechanical ventilation
NF‐κB - nuclear factor‐κB
PCNA - proliferating cell nuclear antigen
PEEP - positive end‐expiratory pressure
RAS - renin‐angiotensin system
RT‐PCR - reverse transcription and polymerase chain reaction
TNFα - tumour necrosis factor α
VILI - ventilator‐induced lung injury
Footnotes
This study was supported by grants from the National Taiwan University Hospital (NTUH‐94S97) and the National Science Council (NSC93‐2314‐B‐002‐223), Taiwan.
Competing interests: None.
A detailed description of the Methods is available in the online supplement at http://thorax.bmj.com/supplemental
References
- 1.Ware L B, Matthay M A. The acute respiratory distress syndrome. N Engl J Med 20003421334–1349. [DOI] [PubMed] [Google Scholar]
- 2.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 20003421301–1308. [DOI] [PubMed] [Google Scholar]
- 3.Tremblay L N, Slutsky A S. Ventilator‐induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians 1998110482–488. [PubMed] [Google Scholar]
- 4.Kawano T, Mori S, Cybulsky M.et al Effect of granulocyte depletion in a ventilated surfactant‐depleted lung. J Appl Physiol 19876227–33. [DOI] [PubMed] [Google Scholar]
- 5.Tremblay L, Valenza F, Ribeiro S P.et al Injurious ventilatory strategies increase cytokines and c‐fos m‐RNA expression in an isolated rat lung model. J Clin Invest 199799944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Held H D, Boettcher S, Hamann L.et al Ventilation‐induced chemokine and cytokine release is associated with activation of nuclear factor‐κB and is blocked by steroids. Am J Respir Crit Care Med 2001163711–716. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Ruiz‐Ortega M, Lorenzo O.et al Inflammation and angiotensin II. Int J Biochem Cell Biol 200335881–900. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz‐Ortega M, Ruperez M, Lorenzo O.et al Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 20026212–22. [DOI] [PubMed] [Google Scholar]
- 9.Esteban V, Lorenzo O, Ruperez M.et al Angiotensin II, via AT1 and AT2 receptors and NF‐κB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J Am Soc Nephrol 2004151514–1529. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz‐Ortega M, Lorenzo O, Ruperez M.et al Systemic infusion of angiotensin II into normal rats activates nuclear factor‐κB and AP‐1 in the kidney: role of AT1 and AT2 receptors. Am J Pathol 20011581743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimmeler S, Rippmann V, Weiland U.et al Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res 199781970–976. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Alam G, Zagariya A.et al Apoptosis of lung epithelial cells in response to TNF‐alpha requires angiotensin II generation de novo. J Cell Physiol 2000185253–259. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Zagariya A, Ibarra‐Sunga O.et al Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 1999276L885–L889. [DOI] [PubMed] [Google Scholar]
- 14.Marshall R P, Gohlke P, Chambers R C.et al Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol 2004286L156–L164. [DOI] [PubMed] [Google Scholar]
- 15.Imai Y, Kuba K, Rao S.et al Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature 2005436112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehead T C, Zhang H, Mullen B.et al Effect of mechanical ventilation on cytokine response to intratracheal lipopolysaccharide. Anesthesiology 200410152–58. [DOI] [PubMed] [Google Scholar]
- 17.Ricard J D, Dreyfuss D, Saumon G. Production of inflammatory cytokines in ventilator‐induced lung injury: a reappraisal. Am J Respir Crit Care Med 20011631176–1180. [DOI] [PubMed] [Google Scholar]
- 18.Torre D, Minoja G, Maraggia D.et al Effect of recombinant IL‐1 beta and recombinant gamma interferon on septic acute lung injury in mice. Chest 19941051241–1245. [DOI] [PubMed] [Google Scholar]
- 19.Goldblum S E, Wu K M, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol 1985591978–1985. [DOI] [PubMed] [Google Scholar]
- 20.Fink M P, O'Sullivan B P, Menconi M J.et al A novel leukotriene B4‐receptor antagonist in endotoxin shock: a prospective, controlled trial in a porcine model. Crit Care Med 1993211825–1837. [DOI] [PubMed] [Google Scholar]
- 21.Schuster M, Tschernig T, Krug N.et al Lymphocytes migrate from the blood into the bronchoalveolar lavage and lung parenchyma in the asthma model of the brown Norway rat. Am J Respir Crit Care Med 2000161558–566. [DOI] [PubMed] [Google Scholar]
- 22.Bechara R I, Pelaez A, Palacio A.et al Angiotensin II mediates glutathione depletion, transforming growth factor‐beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 2005289L363–L370. [DOI] [PubMed] [Google Scholar]
- 23.Haddad el B, McCluskie K, Birrell M A.et al Differential effects of ebselen on neutrophil recruitment, chemokine, and inflammatory mediator expression in a rat model of lipopolysaccharide‐induced pulmonary inflammation. J Immunol 2002169974–982. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Quinton L J, Bagby G J.et al Interferon‐gamma enhances the pulmonary CXC chemokine response to intratracheal lipopolysaccharide challenge. J Infect Dis 200318762–69. [DOI] [PubMed] [Google Scholar]
- 25.Ledbetter S, Kurtzberg L, Doyle S.et al Renal fibrosis in mice treated with human recombinant transforming growth factor‐beta2. Kidney Int 2000582367–2376. [DOI] [PubMed] [Google Scholar]
- 26.Dreyfuss D, Saumon G. Ventilator‐induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998157294–323. [DOI] [PubMed] [Google Scholar]
- 27.Campbell D J, Habener J F. Hybridization in situ studies of angiotensinogen gene expression in rat adrenal and lung. Endocrinology 1989124218–222. [DOI] [PubMed] [Google Scholar]
- 28.Cassis L, Shenoy U, Lipke D.et al Lung angiotensin receptor binding characteristics during the development of monocrotaline‐induced pulmonary hypertension. Biochem Pharmacol 19975427–31. [DOI] [PubMed] [Google Scholar]
- 29.Entzeroth M, Hadamovsky S. Angiotensin II receptors in the rat lung are of the AII‐1 subtype. Eur J Pharmacol 1991206237–241. [DOI] [PubMed] [Google Scholar]
- 30.Wolf G, Wenzel U, Burns K D.et al Angiotensin II activates nuclear transcription factor‐κB through AT1 and AT2 receptors. Kidney Int 2002611986–1995. [DOI] [PubMed] [Google Scholar]
- 31.Takase O, Marumo T, Imai N.et al NF‐kappaB‐dependent increase in intrarenal angiotensin II induced by proteinuria. Kidney Int 200568464–473. [DOI] [PubMed] [Google Scholar]
- 32.Cowling R T, Gurantz D, Peng J.et al Transcription factor NF‐κB is necessary for up‐regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor‐alpha or interleukin‐1 beta. J Biol Chem 20022775719–5724. [DOI] [PubMed] [Google Scholar]
- 33.Ohkubo T, Chapman N, Neal B.et al Effects of an angiotensin‐converting enzyme inhibitor‐based regimen on pneumonia risk. Am J Respir Crit Care Med 20041691041–1045. [DOI] [PubMed] [Google Scholar]
- 34.Esteban V, Ruperez M, Vita J R.et al Effect of simultaneous blockade of AT1 and AT2 receptors on the NF‐κB pathway and renal inflammatory response. Kidney Int Suppl 200386S33–S38. [DOI] [PubMed] [Google Scholar]
- 35.Macari D, Whitebread S, Cumin F.et al Renal actions of the angiotensin AT2 receptor ligands CGP 42112 and PD 123319 after blockade of the renin‐angiotensin system. Eur J Pharmacol 199425927–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.