Abstract
Background
The feasibility of anatomical lobectomy in patients with bronchial carcinoma in an area of severe heterogeneous emphysema whose respiratory reserve is outside operability guidelines has previously been confirmed. A review was undertaken to determine whether this approach is justified by long‐term survival.
Methods
A single surgeon's 8 year experience of 118 consecutive patients (74 men) of median age 70 years (range 45–84) who underwent upper lobectomy for pathological stage I non‐small cell lung cancer (NSCLC) was reviewed. The preoperative characteristics, perioperative course and survival of the 27 cases with severe heterogeneous emphysema of apical distribution and a predicted postoperative forced expiratory volume in 1 s (ppoFEV1) of <40% (lobarLVRS group) were compared with the remaining 91 cases with a ppoFEV1 of >40% (control group).
Results
Postoperative mortality was 1 of 27 in the lobarLVRS group and 2 of 91 in the control group (p = NS). Five‐year survival in the lobarLVRS group was 35% compared with 65% in the control group without concomitant severe emphysema (p = 0.001), although rates of tumour recurrence were similar.
Conclusions
Long‐term survival after lobarLVRS for stage I lung cancer is limited by physiological rather than oncological factors. However, outcomes are still better than those reported for any other modality of treatment in this group of high‐risk patients. This finding justifies the decision to offer lobectomy in these selected cases.
When possible, anatomical lobectomy is the procedure of choice in stage I non‐small cell lung cancer (NSCLC).1 However, reports and available guidelines for operability in the management of these patients state that patients with a predicted postoperative forced expiratory volume in 1 s (ppoFEV1) of <40% are at risk of complications if surgical resection is undertaken.2,3 As a result, other alternatives have been suggested for these patients including palliative care, radiation therapy and sublobar resections.4,5,6,7 Increasing experience with surgical treatment of severe emphysema led some authors to apply the principles of lung volume reduction surgery (LVRS) to the management of lung cancer in patients with emphysema.8,9,10 We have previously described the short‐term feasibility of performing a lobectomy in patients with resectable NSCLC located within an area of severe emphysema who would fall outside published guidelines of fitness for surgery.11 Based on the finding that actual postoperative lung function was significantly better than predicted, we coined the phrase “lobar lung volume reduction for cancer” (lobarLRVS). We have continued to use this approach as part of our commitment to improve resection rates,12 and also to provide the best surgical option to patients who may have been previously deemed unfit for a lobectomy. However, we were conscious of the argument that, although feasible, this strategy must be justified by long‐term outcome to gain wider acceptance. We therefore compared the long‐term outcomes of patients with heterogeneous emphysema of apical distribution and impaired respiratory reserve (ppoFEV1 <40%) with those without severe emphysema who underwent upper lobectomy and systematic nodal dissection for stage I NSCLC.
Methods
Over an 8 year period (April 1997 to March 2005), 118 patients (74 men) underwent upper lobectomy with systematic lymph node dissection for stage I NSCLC under a single surgeon's care. Twenty‐seven (23%) had severe heterogeneous emphysema of apical distribution with a predicted ppoFEV1 of <40% (lobarLVRS group). Their perioperative course, tumour recurrence and survival were compared with the remaining 91 (77%) patients (control group).
Preoperative characteristics
The median age of the patients was 69 years (range 45–84). Twenty‐eight patients (24%) were aged >75 years. The median FEV1 was 70% predicted (range 17–118) with a median ppoFEV1 of 54% (range 14–99). The median preoperative carbon monoxide transfer factor (Tlco) in the lobarLVRS group was 47% predicted (range 32–97). The preoperative characteristics and operative details of the two groups are shown in table 1.
Table 1 Preoperative characteristics of the two groups expressed as median (range) or n (%).
| LobarLVRS (N = 27) | Lobectomy (N = 91) | p Value | |
|---|---|---|---|
| M:F | 20:7 | 54:37 | NS |
| Age (years) | 69 (51–79) | 70 (45–84) | NS |
| >75 years | 7 (26%) | 21 (23%) | NS |
| FEV1 (% predicted) | 45 (19–54) | 77 (53–118) | 0.001 |
| ppoFEV1 (%) | 34 (14–39) | 61 (41–99) | 0.001 |
| Right:left | 13:14 | 52:39 | NS |
| Preoperative Tlco (% predicted) | 47 (32–97) | NA | |
| Q score | 7.5 (1.5–13) | NA | |
| Body mass index | 23 (18–30) | 24 (18–33) | NS |
FEV1, forced expiratory volume in 1 s; ppoFEV1, predicted postoperative forced expiratory volume in 1 s; LVRS, lung volume reduction surgery; Tlco, carbon monoxide transfer factor.
Selection criteria
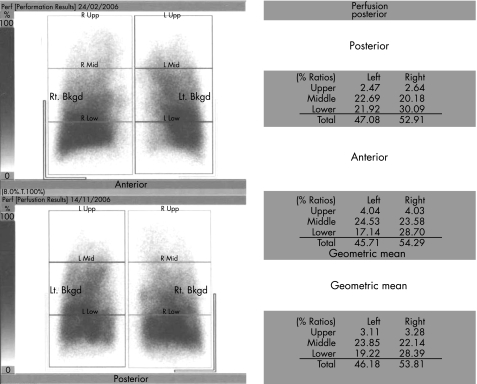
Resectability was defined by a staging CT scan with a negative cervical mediastinoscopy if lymph nodes were >1 cm in their short axis on the CT scan. We now perform an integrated PET/CT scan but it was not available during the period of the study. The ppoFEV1 was calculated according to a segment counting equation system that we have used since our original report.11 Fitness for surgery for lobectomy was defined by ppoFEV1 >40%. In cases where ppoFEV1 was <40%, a lung perfusion scan with regional distribution was performed to confirm that the cancer was located within an area of emphysema (fig 1).13 Based on the results of the perfusion scan, we defined a Q score as the fraction of perfusion of the affected lung region. A Q score of <10 (<10% of total) would define a lung region hypoperfused due to emphysema. However, we do not use the Q score to calculate ppoFEV1 as it is a non‐anatomical method and the three zones do not correspond with the anatomical lobes. The selection criteria for these patients followed our standard selection for LVRS.14 Thus, patients with a ppoFEV1 <40% and homogeneous emphysema on perfusion scintigraphy were excluded and received non‐surgical treatment because of their high risk of perioperative death.15
Figure 1 Perfusion scan showing bilateral apical perfusion defects consistent with emphysematous regions. The Q score is the fraction of perfusion of the apical zone divided by the total lung perfusion.
All patients were followed up in the surgical outpatient clinic and survival was confirmed via a national registry.
Statistical analysis
The data are presented as median (range) and number (%) unless stated otherwise. Univariate analysis was performed using the χ2 test for qualitative data and the Wilcoxon rank test for quantitative data. Postoperative survival was plotted according to the Kaplan‐Meier method and any difference in survival between the groups was evaluated with the log‐rank test. Statistical significance was defined by p values <0.05 throughout the study.
Results
There were 65 right upper lobectomies and 53 left upper lobectomies with no differences in distribution between the two groups (table 1). In the lobarLVRS and control groups, histological examination revealed adenocarcinoma in 6 (22%) and 36 cases (40%), squamous cell carcinoma in 17 (63%) and 42 cases (46%), and large cell carcinoma/undifferentiated in 4 (15%) and 13 cases (14%), respectively. Four patients (15%) in the lobarLVRS group and 21 (23%) in the control group had pathological stage Ia disease while 23 and 70 had stage Ib disease in the two groups.
Postoperative course
There were three postoperative deaths (2.5%). In the lobarLVRS group a 76‐year‐old man (ppoFEV1 20%) died of MRSA pneumonia 26 days after a right upper lobectomy. In the control group two patients (ppoFEV1 52% and 41%) died of myocardial infarction within 48 h of surgery. The median duration of chest drainage was 5 days (range 1–36) and median hospital stay was 7.5 days (range 3–63). There were no differences between the two groups (table 2).
Table 2 Perioperative results expressed as median (25–75% interquartile range) or n (%).
| LobarLVRS (N = 27) | Lobectomy (N = 91) | p Value | |
|---|---|---|---|
| Hospital mortality | 1 (3.7%) | 2 (2.2%) | NS |
| Hospital stay (days) | 8 (5.5–13) | 7 (6–11) | NS |
| Duration of drainage (days) | 5 (3.5–10) | 5 (4–7) | NS |
| Stage Ia:Ib | 4:23 | 21:70 | NS |
LVRS, lung volume reduction surgery.
Survival
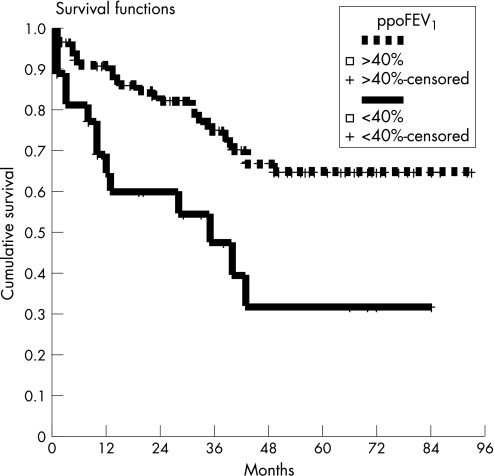
At the end of the study 26 patients (76.5%) were alive. With a median follow‐up of 57 months (range 1–95), the mean (SE) overall 5 year survival was 58.3 (5)%. There were no significant differences in survival (fig 2), disease‐free survival or locoregional recurrences between the two groups (table 3).
Figure 2 Survival according to the Kaplan‐Meier method of the lobar lung volume reduction surgery (lobarLVRS) group vs lobectomy group (p = 0.001, log rank test).
Table 3 Long‐term results expressed as median (25–75% interquartile range) or n (%).
| LobarLVRS (N = 27) | Lobectomy (N = 91) | p Value | |
|---|---|---|---|
| Follow‐up (months) | 57 (29–72) | 57 (36–75) | NS |
| Total recurrence | 6 (22%) | 16 (18%) | NS |
| Locoregional recurrence | 3 (11%) | 10 (11%) | NS |
| Mean (SE) actuarial 3 year survival* | 48 (11)% | 75 (4)% | 0.001 |
| Mean (SE) actuarial 5 year survival* | 35 (11)% | 65 (5)% | 0.001 |
LVRS, lung volume reduction surgery.
*According to Kaplan‐Meier method.
Discussion
In recent years we have achieved a better understanding of the consequences of surgery for end‐stage emphysema and the management of its complications. This has led doctors to aim to extend the indications for surgery for NSCLC to patients previously deemed unfit for surgery due to concomitant severe emphysema.8,9,10
To date, the limited data available include mostly patients undergoing sublobar resections in areas of emphysema7 and feasibility studies of lobectomy for NSCLC on an emphysematous lung.11,16,17 The rationale for performing sublobar resections for early stage NSCLC is based on the principle that surgery only achieves control of local disease that can be achieved with limited removal of lung parenchyma, therefore minimising morbidity/mortality.7 We have previously reported a case‐match comparative study between anatomical segmentectomy and lobectomy for early NSCLC in compromised patients and obtained similarly good outcomes in both groups.5 The difference from the current report is that most of the patients in that study suffered from cancer located in the lower lobes. However, reasonable doubts still remain about the oncological value of sublobar or non‐anatomical resections for NSCLC.1
Our report of “lobarLVRS”11 did concur with other authors in that lobectomy for carcinoma in patients with heterogeneous emphysema with severely impaired respiratory reserve is feasible with acceptable mortality and with preservation/improvement of the respiratory function after surgery.8,16,17 However, there are very few data on the long‐term outcomes of these patients to determine whether this aggressive approach is justified by survival. Cerfolio et al8 reported a 5 year survival in patients with stage I disease of 54% which compares favourably with our series. In addition, in an extensive series of 106 patients (73 undergoing lobectomy), Magdeleinat et al18 reported a hospital mortality rate of 8.5% with a 5 year survival of 33% (44% in stage I compared with 35% in our series). We note with interest that, in their report, spirometric values were better and the patients were younger than in our series. Birim et al19 reported a 5 year survival of 36% after surgery for early carcinoma in a high‐risk group (according to the Charlton comorbidity index).
We did not find significant differences in terms of cancer recurrence between the groups. Our findings concur with those of Sekine and colleagues who reported an increase in non‐cancer related death in patients undergoing pulmonary resection for cancer with concomitant chronic obstructive pulmonary disease (COPD).20
It is also important to take into consideration the natural history of patients with severe emphysema in the absence of lung cancer. Although the data are limited in terms of follow‐up, some of the recent reports of patients undergoing LVRS for heterogeneous emphysema did include long‐term survival. The National Emphysema Treatment Trial Research Group (NETT) reported a 5 year survival of 60% in both surgical and medical groups.21 Other reports are similar with survival rates of 56–71% at 4 or 5 years after LVRS.22,23,24,25,26,27 A recent follow‐on report of the NETT trial estimates survival of around 60% 5 years after LVRS.28 The implication for our series is that the expected survival for the patients in our lobarLVRS group is less than in the control group because of the COPD, so one could not expect similar survival between the groups after surgery.
Another important point to consider when deciding on the therapeutic approach in this group of patients is non‐surgical treatment options. There is very little evidence on the use of radical radiotherapy in medically inoperable patients with lung cancer.29 The subgroup analysis of the CHART trial reported 5 year survival rates of 12–18% depending on the method of delivery of radical radiotherapy.30 Results of survival of non‐randomised studies vary between 0% and 42% at 5 years.29
We acknowledge the limitations of our study. It is the result of a retrospective study and is not randomised. Data and follow‐up were complete in all cases, but information on patients who did not undergo surgery was not available. Also, the use of other preoperative tests such as measurement of carbon monoxide transfer factor and nuclear perfusion scans were not obtained in all patients in the control group so they are not included in the report. The follow‐up protocol does not include routine CT scans to exclude recurrences unless it is indicated by clinical examination, symptoms or new abnormalities on the chest radiograph.
In summary, we have followed on our feasibility report with a long‐term follow‐up study of a cohort of patients undergoing upper lobectomy for stage I lung cancer in an emphysematous lobe who would be suitable for LVRS but not for lobectomy according to guidelines. The long‐term results are affected by death without evidence of cancer recurrence. However, the survival is better than other reported modalities of treatment. This aggressive approach is therefore justified in this group of high‐risk patients. A prospective randomised controlled trial comparing surgery and radical radiotherapy is needed to confirm our conclusions.
Acknowledgements
The authors thank Drs K J West, J Entwistle and M Peake for their help in the clinical management of these patients.
Abbreviations
LVRS - lung volume reduction surgery
NSCLC - non‐small cell lung cancer
ppoFEV1 - predicted postoperative forced expiratory volume in 1 s
Tlco - carbon monoxide transfer factor
Footnotes
Competing interests: None.
References
- 1.Ginsberg R J, Rubinstein L V. Randomized trial of lobectomy versus limited resection for T1N0 non‐small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 199560615–623. [DOI] [PubMed] [Google Scholar]
- 2.British Thoracic Society Guidelines on the selection of patients with lung cancer for surgery. Thorax 20015689–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney D J, Lee T H, Reilly J J.et al Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest 1994105753–759. [DOI] [PubMed] [Google Scholar]
- 4.Albertucci M, DeMeester T R, Rothberg M.et al Surgery and the management of peripheral lung tumors adherent to the parietal pleura. J Thorac Cardiovasc Surg 19921038–12. [PubMed] [Google Scholar]
- 5.Martin‐Ucar A E, Nakas A, Pilling J E.et al A case‐matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high‐risk patients. Eur J Cardiothorac Surg 200527675–679. [DOI] [PubMed] [Google Scholar]
- 6.Linden P A, Bueno R, Colson Y L.et al Lung resection in patients with preoperative FEV1 <35% predicted. Chest 20051271984–1990. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker D J. Lung cancer. 6: The case for limited surgical resection in non‐small cell lung cancer, Thorax 200358639–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerfolio R J, Allen M S, Trastek V F.et al Lung resection in patients with compromised pulmonary function. Ann Thorac Surg 199662348–351. [PubMed] [Google Scholar]
- 9.McKenna R J, Jr, Fischel R J, Brenner M.et al Combined operations for lung volume reduction surgery and lung cancer. Chest 1996110885–888. [DOI] [PubMed] [Google Scholar]
- 10.Korst R J, Ginsberg R J, Ailawadi M.et al Lobectomy improves ventilatory function in selected patients with severe COPD. AnnThorac Surg199866898–902. [DOI] [PubMed] [Google Scholar]
- 11.Edwards J G, Duthie D J, Waller D A. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax 200156791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin‐Ucar A E, Waller D A, Atkins J L.et al The beneficial effects of specialist thoracic surgery on the resection rate for non‐small cell lung cancer. Lung Cancer 200446227–232. [DOI] [PubMed] [Google Scholar]
- 13.Wang S C, Fischer K C, Slone R M.et al Perfusion scintigraphy in the evaluation for lung volume reduction surgery: correlation with clinical outcome. Radiology 1997205243–248. [DOI] [PubMed] [Google Scholar]
- 14.Oey I F, Waller D A, Bal S.et al Lung volume reduction surgery: a comparison of the long term outcome of unilateral vs. bilateral approaches. Eur J Cardiothorac Surg 200222610–614. [DOI] [PubMed] [Google Scholar]
- 15.National Emphysema Treatment Trial Research Group ( N E T T ) Patients at high risk of death after lung‐volume –reduction‐surgery. N Engl J Med 20013451075–1083. [DOI] [PubMed] [Google Scholar]
- 16.DeMeester S R, Patterson G A, Sundaresan R S.et al Lobectomy combined with volume reduction for patients with lung cancer and advanced emphysema. J Thorac Cardiovasc Surg 1998115681–688. [DOI] [PubMed] [Google Scholar]
- 17.Pompeo E, De Dominicis E, Ambrogi V.et al Quality of life after tailored combined surgery for stage I non‐small‐cell lung cancer and severe emphysema. Ann Thorac Surg 2003761821–1827. [DOI] [PubMed] [Google Scholar]
- 18.Magdeleinat P, Seguin A, Alifano M.et al Early and long‐term results of lung resection for non‐small‐cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005271099–1105. [DOI] [PubMed] [Google Scholar]
- 19.Birim O, Kappetein A P, Goorden T.et al Proper treatment selection may improve survival in patients with clinical early‐stage nonsmall cell lung cancer. Ann Thorac Surg 2005801021–1027. [DOI] [PubMed] [Google Scholar]
- 20.Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long‐term survival of patients undergoing surgery for NSCLC. Lung Cancer 20023795–101. [DOI] [PubMed] [Google Scholar]
- 21.Fishman A, Martinez F, Naunheim K.et al National Emphysema Treatment Trial Research Group. A randomized trial comparing lung‐volume‐reduction surgery with medical therapy for severe emphysema. N Engl J Med 20033482059–2073. [DOI] [PubMed] [Google Scholar]
- 22.Yusen R D, Lefrak S S, Gierada D S.et al A prospective evaluation of lung volume reduction surgery in 200 consecutive patients. Chest 20031231026–1037. [DOI] [PubMed] [Google Scholar]
- 23.Ciccone A M, Meyers B F, Guthrie T J.et al Long‐term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003125513–525. [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto T, Teschler H, Hillejan L.et al Long‐term results of lung volume reduction surgery. Eur J Cardiothorac Surg 200221483–488. [DOI] [PubMed] [Google Scholar]
- 25.Juan Samper G, Ramon Capilla M, Canto Armengol A.et al Four‐year results after lung volume reduction surgery for emphysema. Arch Bronconeumol 200440443–448. [PubMed] [Google Scholar]
- 26.Appleton S, Adams R, Porter S.et al Sustained improvements in dyspnea and pulmonary function 3 to 5 years after lung volume reduction surgery. Chest 20031231838–1846. [DOI] [PubMed] [Google Scholar]
- 27.Pompeo E, Mineo T C, Pulmonary Emphysema Research Group Long‐term outcome of staged versus one‐stage bilateral thoracoscopic reduction pneumoplasty. Eur J Cardiothorac Surg 200221627–633. [DOI] [PubMed] [Google Scholar]
- 28.Naunheim K S, Wood D E, Mohsenifar M D.et al Long‐term follow‐up of patients receiving lung‐volume‐reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 200682431–443. [DOI] [PubMed] [Google Scholar]
- 29.Rowell N P, Williams C J. Radical radiotherapy for stage I/II non‐small cell lung cancer in patients not sufficiently fit or declining surgery (medically inoperable). Cochrane Database Syst Rev 2001(2)CD002935. [DOI] [PubMed]
- 30.Bentzen S M, Saunders M I, Dische S.et al Updated data from CHART in NSCLC: further analyses. Radiother Oncol 20005586–87. [Google Scholar]