Abstract
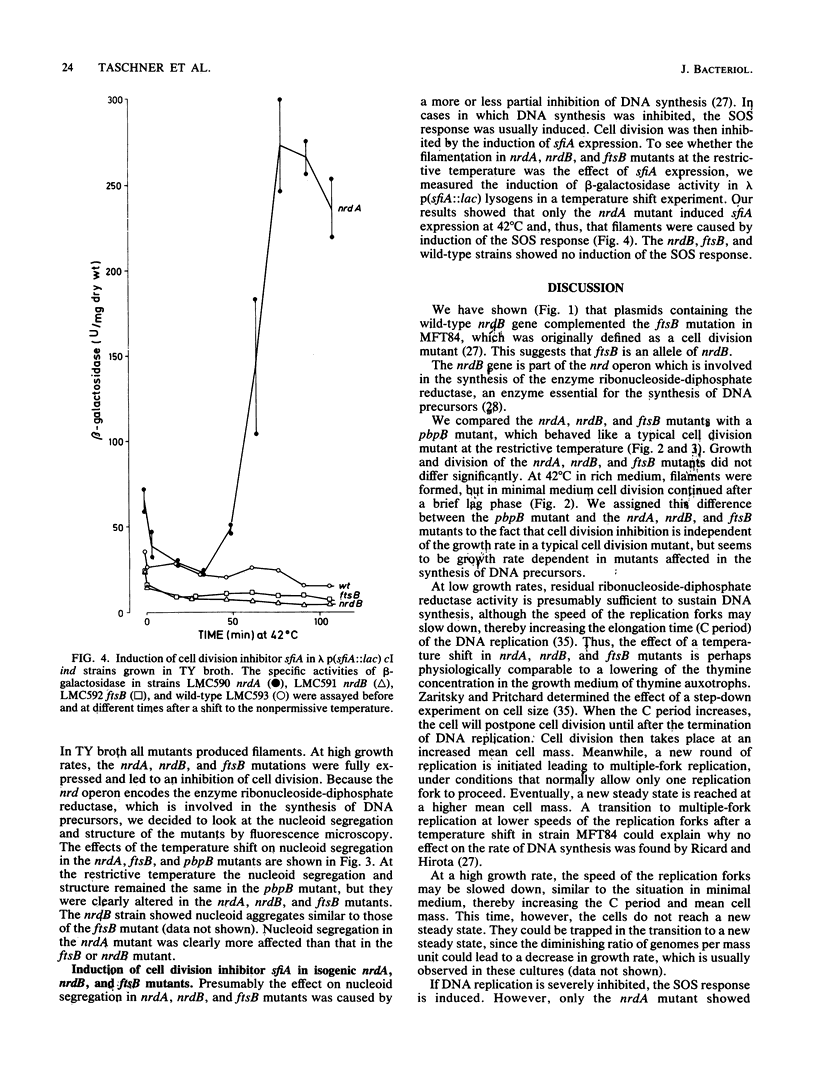
The ftsB gene of Escherichia coli is believed to be involved in cell division. In this report, we show that plasmids containing the nrdB gene could complement the ftsB mutation, suggesting that ftsB is an allele of nrdB. We compared changes in the cell shape of isogenic nrdA, nrdB, ftsB, and pbpB strains at permissive and restrictive temperatures. Although in rich medium all strains produced filaments at the restrictive temperature, in minimal medium only a 50 to 100% increase in mean cell mass occurred in the nrdA, nrdB, and ftsB strains. The typical pbpB cell division mutant also formed long filaments at low growth rates. Visualization of nucleoid structure by fluorescence microscopy demonstrated that nucleoid segregation was affected by nrdA, nrdB, and ftsB mutations at the restrictive temperature. Measurements of beta-galactosidase activity in lambda p(sfiA::lac) lysogenic nrdA, nrdB, and ftsB mutants in rich medium at the restrictive temperature showed that filamentation in the nrdA mutant was caused by sfiA (sulA) induction, while filamentation in nrdB and ftsB mutants was sfiA independent, suggesting an SOS-independent inhibition of cell division.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. S., Filip C. C., Gustafson R. A., Allen R. G., Walker J. R. Regulation of bacterial cell division: genetic and phenotypic analysis of temperature-sensitive, multinucleate, filament-forming mutants of Escherichia. J Bacteriol. 1974 Mar;117(3):978–986. doi: 10.1128/jb.117.3.978-986.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burton P., Holland I. B. Two pathways of division inhibition in UV-irradiated E. coli. Mol Gen Genet. 1983;190(1):128–132. doi: 10.1007/BF00330334. [DOI] [PubMed] [Google Scholar]
- Carlson J., Fuchs J. A., Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of ribonucleoside diphosphate reductase synthesis in Escherichia coli: increased enzyme synthesis as a result of inhibition of deoxyribonucleic acid synthesis. J Bacteriol. 1977 Apr;130(1):107–113. doi: 10.1128/jb.130.1.107-113.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O. A mutant of Escherichia coli defective in ribonucleosidediphosphate reductase. 2. Characterization of the enzymatic defect. Eur J Biochem. 1973 Feb 1;32(3):457–462. [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O., Warner H. R., Reichard P. Defective gene product in dnaF mutant of Escherichia coli. Nat New Biol. 1972 Jul 19;238(81):69–71. doi: 10.1038/newbio238069a0. [DOI] [PubMed] [Google Scholar]
- Fuchs J. A., Neuhard J. A mutant of Escherichia coli defective in ribonucleosidediphosphate reductase. 1. Isolation of the mutant as a deoxyuridine auxotroph. Eur J Biochem. 1973 Feb 1;32(3):451–456. doi: 10.1111/j.1432-1033.1973.tb02627.x. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke P. D., Fuchs J. A. Requirement of protein synthesis for the induction of ribonucleoside diphosphate reductase mRNA in Escherichia coli. Mol Gen Genet. 1984;193(2):327–331. doi: 10.1007/BF00330689. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. Effect of suppressors of SOS-mediated filamentation on sfiA operon expression in Escherichia coli. J Bacteriol. 1983 Jan;153(1):169–175. doi: 10.1128/jb.153.1.169-175.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Norris V. SOS-independent coupling between DNA replication and cell division in Escherichia coli. J Bacteriol. 1986 Jan;165(1):66–71. doi: 10.1128/jb.165.1.66-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren B., Fuchs J. A. Characterization of the ftsB gene as an allele of the nrdB gene in Escherichia coli. J Bacteriol. 1987 Jan;169(1):14–18. doi: 10.1128/jb.169.1.14-18.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem. 1976 Jan;24(1):24–33. doi: 10.1177/24.1.943439. [DOI] [PubMed] [Google Scholar]
- Ludtke D., Larson T. J., Beck C., Boos W. Only one gene is required for the glpT-dependent transport of sn-glycerol-3-phosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):540–547. doi: 10.1007/BF00337962. [DOI] [PubMed] [Google Scholar]
- Platz A., Sjöberg B. M. Construction and characterization of hybrid plasmids containing the Escherichia coli nrd region. J Bacteriol. 1980 Aug;143(2):561–568. doi: 10.1128/jb.143.2.561-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz A., Sjöberg B. M. Mutationally altered ribonucleotide reductase from Escherichia coli: characterization of mutations isolated on multicopy plasmids. J Bacteriol. 1984 Dec;160(3):1010–1016. doi: 10.1128/jb.160.3.1010-1016.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., Valkendurg J. A., Pas E., Taschner P. E., Huls P., Wientjes F. B. Physiological and geometrical conditions for cell division in Escherichia coli. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):131–138. doi: 10.1016/s0769-2609(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Yamada M., Takeda Y., Okamoto K., Hirota Y. Physical map of the nrdA-nrdB-ftsB-glpT region of the chromosomal DNA of Escherichia coli. Gene. 1982 Jun;18(3):309–318. doi: 10.1016/0378-1119(82)90169-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]




