Abstract
Background
The control of tuberculosis (TB) is founded on early case detection and complete treatment of disease. In the UK, TB is concentrated in subgroups of the population in large urban centres. The impact of homelessness, imprisonment and problem drug use on TB control in London is reviewed.
Methods
A cohort study was undertaken of all patients with TB in Greater London to determine the point prevalence of disease in different groups and to examine risk factors for smear positivity, drug resistance, treatment adherence, loss to follow‐up and use of directly observed therapy (DOT).
Results
Data were collected on 97% (1941/1995) of eligible patients. The overall prevalence of TB was 27 per 100 000. An extremely high prevalence of TB was seen in homeless people (788/100 000), problem drug users (354/100 000) and prisoners (208/100 000). Multivariate analysis showed that problem drug use was associated with smear positive disease (OR 2.2, p<0.001), being part of a known outbreak of drug resistant TB (OR 3.5, p = 0.001) and loss to follow‐up (OR 2.7, p<0.001). Imprisonment was associated with being part of the outbreak (OR 10.3, p<0.001) and poor adherence (OR 3.9, p<0.001). Homelessness was associated with infectious TB (OR 1.6, p = 0.05), multidrug resistance (OR 2.1, p = 0.03), poor adherence (OR 2.5, p<0.001) and loss to follow‐up (OR 3.8, p<0.001). In London, homeless people, prisoners and problem drug users collectively comprise 17% of TB cases, 44% of smear positive drug resistant cases, 38% of poorly compliant cases and 44% of cases lost to follow‐up. 15% of these patients start treatment on DOT but 46% end up on DOT.
Conclusions
High levels of infectious and drug resistant disease, poor adherence and loss to follow‐up care indicate that TB is not effectively controlled among homeless people, prisoners and problem drug users in London.
In the developed world, tuberculosis (TB) is increasingly concentrated in subgroups of the population in large urban centres. TB is a major public health problem in London, where there was an 11% increase in new reported cases between 2004 and 2005 and now accounts for 45% of all cases reported in England.1 Rates of disease have doubled from 21.2 per 100 000 per year in 1987 to 47 per 100 000 per year in 2005.1 A large outbreak of drug resistant tuberculosis in London, with over 220 linked cases, has disproportionately involved problem drug users, prisoners and the homeless, highlighting weak control among these groups.2,3
Tuberculosis control is based on early case detection and ensuring patients complete at least 6 months of regular treatment.4 Failure to do this can lead to increased disease transmission, the development of drug resistance and relapse. Poor adherence is a major barrier to successful treatment.5 In many countries this has led to directly observed therapy (DOT) becoming the accepted standard of care for TB.6 There is a lack of randomised controlled trial evidence to support universal DOT in low prevalence settings.7 In the UK, DOT is recommended for patients who have been or are likely to be poorly adherent;4,8 however, there are limited data on risk factors for poor adherence and on how DOT is used in practice in the UK.
Homelessness, problem drug use and imprisonment affect the ability of patients to access health care and to take treatment. TB is known to be common in the homeless, 9,10 but the extent of the problem in prisoners and drug users and the effect of these social issues on adherence, loss to follow‐up, infectiousness and drug resistance has not been adequately described. Levels of imprisonment, drug use and homelessness are high in London with an estimated 10 000 single homeless people living on the streets or in hostels,11,12 70 000 problem drug users13 and over 5000 prisoners at any one time.14 We conducted a study including all patients with TB in London to describe the impact of homelessness, imprisonment and problem drug use on control of the disease.
Methods
Study design
A cohort study was undertaken of all patients with TB living in London who were or should have been on treatment on 1 July 2003. Eligible patients were identified from the London TB register and local clinic records. Patients' case managers used clinic and hospital records and their knowledge of the patient to complete data collection forms at baseline and again at 12 months. Cases subsequently found not to have TB were excluded from the study.
Homelessness was defined as living in direct access hostels or rough sleeping ever or during the current treatment episode. Imprisonment was defined as any period of incarceration during the current treatment episode. Problem drug use was defined as injecting drug use or long duration/regular use of opiates, cocaine and/or amphetamines.15
Drug resistance was divided into multidrug resistance (resistant to at least isoniazid and rifampicin), isonaizid resistant strains that were part of the London outbreak (defined as patients resident in London at the time of their diagnosis with isolates of Mycobacterium tuberculosis resistant to isoniazid that had the outbreak restriction fragment length polymorphism (RFLP) pattern)6 and isoniazid resistant strains that were not part of the outbreak. Smear positivity related to status at diagnosis. The main outcomes were poor adherence, loss to follow‐up and management with DOT. DOT was defined as treatment being observed by a healthcare worker or other responsible adult. We measured adherence during the first 2 months of treatment because the risk of developing resistance is greatest when the bacterial load is high. Poorly adherent patients were defined as those who admitted poor adherence; had inconsistent pill counts; negative urine tests; or who were switched to DOT or admitted to hospital due to poor adherence. Loss to follow‐up was defined as the patient being out of contact with services for at least 2 months without medication during the first 6 months of treatment. We also collected data on age, sex, foreign birth, ethnicity, problem alcohol use, mental health problems and previous TB.
Analysis of data
Disease prevalence per 100 000 population on 1 July 2003 (and 95% confidence intervals based on the Poisson distribution) was calculated for homeless people, problem drug users and prisoners and compared with prevalence in different ethnic groups and in foreign born and UK born populations. Denominator data on the size of the populations at risk were obtained from published sources.10,11,12,16
We assessed the relationships between variables using univariate and multivariate analyses. Logistic regression analysis was used to calculate univariate odds ratios (ORs), 95% confidence intervals and p values. Multiple logistic regression models (backwards elimination) were used to control for confounding using robust standard errors to account for clustering at the clinic level.17,18,19 All analyses were performed using STATA Version 9 (STATA Corp, College Station, Texas, USA).
Results
There were 1995 eligible patients giving an overall point prevalence on 1 July 2003 of 27.1 per 100 000 (95% CI 25.9 to 28.3, table 1). Baseline data were collected for 97% (1941/1995) of eligible patients; follow‐up data were available for 95% (1841/1941) of these. The prevalence of TB was 788 per 100 000 (95% CI 624 to 982) in the homeless, 354 per 100 000 (95% CI 311 to 401) in problem drug users and 208 per 100 000 (95% CI 104 to 373) in prisoners. The prevalence was 80 per 100 000 (95% CI 76 to 84) in foreign born individuals and 148 per 100 000 (95% CI 131 to 165) in recent migrants with <1 year in the UK (table 1).
Table 1 Prevalence of TB in different population groups.
| Patient characteristics | Number of cases | Population denominator | Prevalence per 100 000 (95% CI) |
|---|---|---|---|
| Overall | 1941 | 717209112 | 27.1 (25.9 to 28.3) |
| Age 0–14 | 92 | 136537712 | 6.7 (5.4 to 8.3) |
| Age 15–29 | 718 | 163996312 | 43.8 (40.6 to 47.1) |
| Age 30–59 | 919 | 299230512 | 30.7 (28.8 to 33.8) |
| Age 60+ | 203 | 117444612 | 17.3 (15.0 to 19.8) |
| Male | 1064 | 346879312 | 30.7 (28.9 to 32.6) |
| Female | 847 | 370329812 | 22.9 (21.4 to 24.5) |
| Foreign born | 1548 | 194290412 | 79.7 (75.8 to 83.7) |
| UK born | 376 | 522918712 | 7.2 (6.5 to 8.0) |
| Recent migrant (arrived <1 year) | 295 | 20000016 | 147.5 (131.2 to 165.3) |
| White | 303 | 510320312 | 5.9 (5.3 to 6.7) |
| South Asian | 650 | 73363512 | 88.6 (81.9 to 95.7) |
| Black African | 748 | 37893312 | 197.4 (183.5 to 212.1) |
| Black Caribbean | 92 | 34356712 | 27.9 (21.6 to 32.8) |
| Other | 209 | 19323512 | 108.2 (94.0 to 123.9) |
| Problem drug users | 248 | 7000013 | 354.3 (311.4 to 401.2) |
| Prison (on 1 July 2003) | 11 | 527814 | 208.4 (104.1 to 372.9) |
| Living in hostel/on street (on 1 July 2003) | 79 | 1002412 | 788.1 (624.0 to 982.2) |
One hundred and ten patients (6%) were homeless. Of these, 42 (38%) had infectious TB, and 30 of 77 culture confirmed cases (39%) had drug resistant disease including five (6.5%) with multidrug resistant disease. Fifty (46%) were documented as being poorly adherent and 17 (15%) were lost to follow‐up (table 2). Multivariate analysis showed that homelessness was associated with smear positive disease (OR 1.6, 95% CI 1.0 to 2.6, p = 0.05), poor adherence (OR 2.5, 95% CI 1.6 to 3.7 p<0.001) and loss to follow‐up (OR 3.8, 95% CI 2.0 to 7.4, p<0.001). Current or previous homelessness (ever homeless) was associated with multidrug resistant TB (OR 2.1, 95 CI 1.1 to 4.1, p = 0.03; table 3).
Table 2 Demographic, disease‐related and social characteristics.
| Patient characteristics | n | Laboratory findings | Management issues | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Culture confirmed n (%) | Sputum smear positive n (%) | Any drug resistance n (%) | MDRTB n (%) | Isoniazid resistant (non‐outbreak) n (%) | Isoniazid resistant (outbreak) n (%) | Non‐adherent in first 2 months n (%) | Loss to follow‐up within 6 months n (%) | DOT from start of treatment n (%) | DOT ever n (%) | ||
| Overall | 1941 | 1121 (57.8%) | 381 (19.6%) | 234 (20.9%) | 67 (6.0%) | 129 (11.5%) | 38 (3.4%) | 350 (18.0%) | 66 (3.4%) | 177 (9.1%) | 492 (25.3%) |
| Age 0–14 | 92 | 29 (31.5%) | 5 (5.4%) | 6 (20.6%) | 2 (6.9%) | 3 (10.3%) | 1 (3.4%) | 8 (8.7%) | 1 (1.1%) | 34 (37.0%) | 56 (60.9%) |
| Age 15–29 | 718 | 444 (61.8%) | 158 (22.0%) | 97 (21.8%) | 27 (6.1%) | 57 (12.8%) | 13 (2.9%) | 138 (19.2%) | 31 (4.3%) | 39 (5.4%) | 143 (19.9%) |
| Age 30–59 | 919 | 536 (58.3%) | 187 (20.3%) | 116 (21.6%) | 32 (6.0%) | 61 (11.4%) | 23 (4.3%) | 169 (18.4%) | 29 (3.2%) | 78 (8.5%) | 215 (23.4%) |
| Age 60+ | 203 | 108 (53.2%) | 31 (15.3%) | 13 (12.0%) | 5 (4.6%) | 7 (6.5%) | 1 (0.9%) | 34 (16.7%) | 5 (2.5%) | 26 (12.8%) | 76 (37.4%) |
| Male | 1064 | 637 (59.9%) | 231 (21.7%) | 139 (21.8%) | 41 (6.4%) | 74 (11.6%) | 24 (3.8%) | 231 (21.7%) | 49 (4.6%) | 97 (9.1%) | 278 (26.1%) |
| Female | 847 | 467 (55.1%) | 147 (17.4%) | 94 (20.1%) | 26 (5.6%) | 54 (11.6%) | 14 (3.0%) | 115 (13.6%) | 17 (2.0%) | 79 (9.3%) | 210 (24.8%) |
| Foreign born | 1548 | 902 (58.3%) | 227 (14.7%) | 179 (19.8%) | 59 (6.5%) | 106 (11.8%) | 14 (1.6%) | 272 (17.6%) | 47 (3.0%) | 110 (7.1%) | 339 (21.9%) |
| UK born | 376 | 206 (54.8%) | 100 (26.6%) | 52 (25.2%) | 6 (2.9%) | 23 (11.2%) | 23 (11.2%) | 74 (19.7%) | 18 (4.8%) | 67 (17.8%) | 149 (39.6%) |
| Recent migrant (<1 year) | 295 | 172 (58.3%) | 54 (18.3%) | 30 (17.4%) | 10 (5.8%) | 19 (11.0%) | 1 (0.6%) | 50 (16.9%) | 8 (2.7%) | 19 (6.4%) | 53 (18.0%) |
| White | 303 | 188 (62.0%) | 85 (28.1%) | 34 (18.1%) | 6 (3.2%) | 19 (10.0%) | 9 (4.8%) | 70 (23.1%) | 13 (4.3%) | 49 (16.2%) | 115 (38.0%) |
| South Asian | 650 | 339 (52.2%) | 79 (12.2%) | 52 (15.3%) | 16 (4.7%) | 33 (9.7%) | 3 (0.9%) | 84 (12.9%) | 20 (3.1%) | 33 (5.1%) | 124 (19.1%) |
| Black African | 748 | 449 (60.0%) | 150 (20.1%) | 100 (22.3%) | 37 (8.2%) | 59 (13.1%) | 4 (0.9%) | 147 (19.7%) | 24 (3.2%) | 60 (8.0%) | 177 (23.7%) |
| Black Caribbean | 92 | 64 (69.6%) | 33 (35.9%) | 25 (39.1%) | 2 (3.2%) | 6 (9.4%) | 17 (26.6%) | 27 (29.3%) | 3 (3.2%) | 19 (20.7%) | 36 (39.1%) |
| Other | 83 | 48 (57.8%) | 19 (22.9%) | 15 (31.3%) | 5 (10.4%) | 8 (16.7%) | 2 (4.2%) | 12 (14.5%) | 4 (4.8%) | 8 (9.6%) | 20 (24.1%) |
| Previous TB | 202 | 111 (55.0%) | 42 (20.8%) | 47 (42.3%) | 26 (23.4%) | 15 (13.5%) | 6 (5.4%) | 64 (31.7%) | 17 (8.4%) | 28 (13.9%) | 79 (39.1%) |
| PDU | 248 | 176 (71.0%) | 97 (39.1%) | 58 (33.0%) | 11 (6.3%) | 20 (11.4%) | 27 (15.3%) | 116 (46.8%) | 25 (10.0%) | 41 (16.5%) | 119 (48.0%) |
| Alcohol | 156 | 111 (71.2%) | 64 (41.0%) | 34 (30.6%) | 5 (4.5%) | 11 (9.9%) | 18 (16.2%) | 82 (52.6%) | 17 (10.8%) | 33 (21.2%) | 84 (53.8%) |
| Prison during treatment | 74 | 55 (74.3%) | 29 (39.2%) | 30 (54.5%) | 8 (14.5%) | 3 (5.5%) | 19 (25.7%) | 44 (59.5%) | 9 (12.1%) | 15 (20.3%) | 54 (73.0%) |
| Homeless during treatment | 110 | 77 (70.0%) | 42 (38.2%) | 30 (39.0%) | 5 (6.5%) | 13 (16.9%) | 12 (15.6%) | 50 (45.5%) | 17 (15.4%) | 21 (19.1%) | 65 (59.1%) |
| Prison or homeless or PDU | 321 | 221 (68.8%) | 115 (35.8%) | 67 (30.3%) | 14 (6.3%) | 26 (11.8%) | 27 (12.2%) | 133 (41.4%) | 29 (9.0%) | 48 (15.0%) | 146 (45.5%) |
MDRTB, multidrug resistant tuberculosis; DOT, directly observed therapy; PDU, problem drug user.
Table 3 Multivariate associations between patient characteristics and infectivity, drug resistance and treatment adherence: OR (95% CI) with p values.
| Laboratory findings | Management issues | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sputum smear positive | Any drug resistance | MDRTB | Isoniazid resistant (non‐outbreak) | Isoniazid resistant (outbreak) | Non‐adherent in first 2 months | Loss to follow‐up within 6 months | DOT from start of treatment | DOT ever | |
| Age 0–14 | 0.3 (0.1 to 0.8) | 1.0 (0.3 to 3.4) | 0.8 (0.2 to 4.6) | 0.6 (0.2 to 1.4) | 0.6 (0.8 to 4.7) | 9.2 (3.3 to 25.6) | 8.2 (3.2 to 20.6) | ||
| Age 15–29 | 1.3 (1.0 to 1.7) | 1.1 (0.8 to 1.6) | 1.1 (0.7 to 1.7) | 1.2 (0.9 to 1.7) | 1.7 (1 to 2.9) | 0.8 (0.5 to 1.4) | 1.0 (0.8 to 1.3) | ||
| Age 30–59 | 1 (p = 0.008) | 1 (p = 0.22) | 1 (p = 0.41) | 1 (p = 0.32) | 1 (p = 0.24) | 1 (p<0.001) | 1 (p<0.001) | ||
| Age 60+ | 0.7 (0.4 to 1.3) | 0.6 (0.4 to 1.0) | 0.5 (0.3 to 1.2) | 1.1 (0.8 to 1.6) | 1.2 (0.5 to 2.9) | 1.8 (0.8 to 3.7) | 2.7 (1.7 to 4.4) | ||
| Male | 1.1 (0.8 to 1.5) (p = 0.38) | 1.0 (0.7 to 1.4) (p = 0.89) | 1.0 (0.7 to 1.6) (p = 0.90) | 1.6 (1.3 to 2.0) (p<0.001) | 1.9 (1.2 to 3.0) (p = 0.006) | 0.8 (0.6 to 1.2) (p = 0.33) | 0.9 (0.7 to 1.1) (p = 0.21) | ||
| Born UK | 2.8 (1.1 to 7.0) (p = 0.03) | ||||||||
| White | 1 (p<0.001) | 1 (p<0.021) | 1 (p = 0.19) | 1 (p = 0.21) | 1 (p<0.001) | 1 (p = 0.03) | 1 (p = 0.21) | 1 (p<0.13) | 1 (p = 0.26) |
| South Asian | 0.4 (0.3 to 0.6) | 1.0 (0.6 to 1.6) | 1.6 (0.8 to 3.0) | 1.0 (0.5 to 2.1) | 1.1 (0.2 to 6.7) | 0.7 (0.5 to 1.1) | 1.5 (0.7 to 3.0) | 0.4 (0.2 to 0.9) | 0.6 (0.4 to 1.0) |
| Black African | 0.8 (0.5 to 1.1) | 1.3 (0.8 to 2.0) | 2.5 (1.2 to 5.7) | 1.4 (0.7 to 2.6) | 0.8 (0.1 to 7.2) | 1.1 (0.8 to 1.6) | 1.3 (0.7 to 2.6) | 0.5 (0.3 to 1.0) | 0.7 (0.5 to 1.1) |
| Black Caribbean | 1.5 (1.0 to 2.1) | 3.0 (1.2 to 7.7) | 1.6 (0.3 to 10.2) | 1.5(0.5 to 5.2) | 9.7 (2.6 to 35.4) | 1.4 (0.9 to 2.3) | 0.7 (0.3 to 1.9) | 1.0 (0.5 to 2.1) | 0.7 (0.4 to 1.6) |
| Other ethnic | 1.0 (0.7 to 1.4) | 1.9 (1.0 to 3.4) | 2.5 (0.9 to 7.1) | 1.8 (0.7 to 4.2) | 6.1 (1.6 to 23.3) | 0.8 (0.5 to 1.3) | 2.1 (0.9 to 4.9) | 0.7 (0.3 to 1.6) | 0.9 (0.6 to 1.6) |
| Previous TB | 3.0 (1.9 to 4.9) (p<0.001) | 7.8 (4.8 to 12.5) (p<0.001) | |||||||
| Problem drug users | 2.2 (1.6 to 2.9) (p<0.001) | 3.5 (1.6 to 7.7) (p = 0.001) | 2.7 (1.6 to 4.3) (p<0.001) | 1.6 (0.8 to 2.9) (p = 0.17) | 2.0 (1.2 to 3.2) (p = 0.004) | ||||
| Prison in this episode | 3.0 (1.7 to 5.5) (p<0.001) | 10.3 (4.0 to 26.5) (p<0.001) | 3.9 (2.5 to 6.1) (p<0.001) | 5.1 (2.6 to 10.3) (p<0.001) | |||||
| Hostel/street homeless | 1.6 (1.0 to 2.6) (p<0.054) | 2.0 (0.9 to 4.5) (p = 0.09) | 2.5 (1.6 to 3.7) (p<0.001) | 3.8 (2.0 to 7.4) (p<0.001) | 1.6 (0.8 to 3.1) (p = 0.16) | 2.6 (1.7 to 3.9) (p<0.001) | |||
| Ever homeless | 1.6 (1.1 to 2.2) (p<0.015) | 2.1 (1.1 to 4.1) (p = 0.028) | |||||||
| Mental health problem | 2.1 (1.3 to 3.3) (p = 0.003) | 2.1 (1.0 to 4.7) (p<0.049) | 3.1 (1.5 to 6.3) (p = 0.002) | 4.3 (2.5 to 7.3) (p<0.001) | |||||
MDRTB, multidrug resistant tuberculosis; DOT, directly observed therapy.
The table includes adjusted odds ratios for variables that remained in the regression model following backwards elimination.
Two hundred and forty‐eight patients (13%) were problem drug users. Among these, 39% (97) were smear positive, 15% (27) were confirmed as part of the London outbreak, 47% (116) were documented as poorly adherent and 10% (25) were lost to follow‐up (table 2). Multivariate analysis showed that problem drug use was associated with smear positive disease (OR 2.2, 95% CI 1.6 to 2.9, p<0.001), being part of the London outbreak of isoniazid resistant TB (OR 3.5, 95% CI 1.6 to 7.7, p = 0.001) and loss to follow‐up (OR 2.7, 95% CI 1.6 to 4.3, p<0.001; table 3).
Seventy‐four patients (4%) were prisoners at some time during treatment. Of these, 29 (39%) had smear positive disease, 19 (26%) were confirmed as part of the London outbreak, 44 (59%) were poorly adherent and 9 (12%) were lost to follow‐up (table 2). Multivariate analysis showed that imprisonment was associated with being part of the London outbreak (OR 10.3, 95% CI 4.0 to 26.5, p<0.001) and poor adherence (OR 3.9, 95% CI 2.5 to 6.1, p<0.001; table 3).
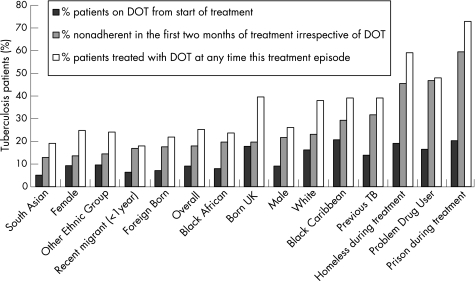
Overall, 350 patients (18%) were non‐adherent in the first 2 months of treatment. South Asians, females, recent migrants and foreign born individuals were most likely to adhere to treatment. Patients least likely to adhere to treatment were homeless people, problem drug users and prisoners. A total of 177 patients (9.1%) started their treatment on DOT with 492 (25.3%) eventually being treated with DOT. Relatively few homeless people, prisoners or drug users started their treatment with DOT, but most ended up being treated with DOT after demonstrating poor adherence (fig 1).
Figure 1 Proportion of patients given directly observed therapy (DOT) from onset, non‐adherent in the first 2 months of treatment and treated with DOT at any point of treatment (ordered by risk of non‐adherence].
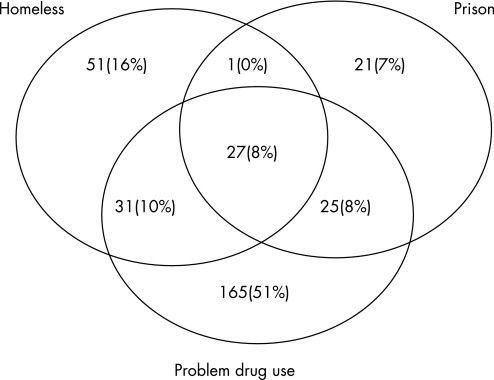
Collectively, problem drug users, homeless people and prisoners made up 17% (321/1941) of TB cases, 38% (133/350) of poorly compliant cases, 44% (29/66) of cases lost to follow‐up, 30% (146/492) of cases ever treated with DOT, 30% (115/381) of smear positive TB cases, 29% (67/234) of drug resistant cases (table 2) and 44% (31/71) of smear positive drug resistant cases. There was a high degree of overlap between homeless people, prisoners and problem drug users (fig 2).
Figure 2 Overlap between prisoners, drug users and homeless people among patients with tuberculosis in London (not to scale).
Discussion
This study shows that TB is a major public health problem in London, and particularly among homeless people, prisoners and problem drug users. These patients have a high prevalence of disease and are often infectious, drug resistant, poorly adherent and lost to follow‐up. They form only 17% of all cases but nearly half of all drug resistant smear positive patients, making a disproportionate impact on control.
Ascertainment of risk factors such as homelessness, drug use and prison history can be difficult. Although we provided clear case definitions, it is likely that we have underestimated the extent of these problems. Estimating the numbers of homeless people and problem drug users in London is also problematic. The reliability of prevalence estimates is also dependent on denominator data. We relied on published estimates of population sizes in London. Measuring poor adherence is notoriously difficult. We relied on hard measures and are therefore likely to have under ascertained poor adherence.
We achieved a high level of completeness for baseline and follow‐up data by working closely with patients' case managers who were highly knowledgeable about their patients. This allowed detailed information on social circumstances that is not normally systematically recorded to be collected with a high degree of accuracy. Collection of data on multiple risk factors enabled confounding to be adequately controlled. The size and pan‐London nature of the study are major strengths. Although the study was confined to London, similar issues are likely to be seen in any cities with large homeless, drug using and prison populations.
Previous studies in the United States have shown that homeless people, prisoners and drug users have high rates of infection, active disease, poor treatment outcomes and to be associated with recent transmission and outbreaks.20,21,22,23 These studies have also shown high levels of overlap between these populations. The contribution of homelessness, imprisonment and problem drug use to TB in the UK context has not been previously described. Data on these factors are not routinely collected. This study confirms these factors as being of major importance in London, quantifies the prevalence of disease in these populations, and shows that homelessness, prison and problem drug use are independent risk factors for drug resistance, smear positivity and poor adherence. Any of these factors either alone or in combination should raise the index of suspicion for TB, alert health professionals to the possibility of drug resistance and infectiousness and the need to instigate measures that will help patients to complete treatment.
Homeless people, problem drug users and prisoners commonly share overcrowded, poorly ventilated spaces leading to a high risk of transmission, as illustrated by the current large outbreak of drug resistant TB in London. In addition to large outbreaks, molecular epidemiological research in the Netherlands has shown intense transmission among drug addicts and homeless people by multiple sources.24 High levels of sputum smear positivity on diagnosis among homeless people, problem drug users and prisoners are likely to indicate delays in case detection. The duration of infectiousness is a key parameter for the transmission of any infectious disease.25 There is a need to evaluate measures to promote early case detection including raising awareness, improving access to services and active case finding measures such as mobile x ray screening.26
In the United States, 86% of homeless patients between 1994 and 2003 were treated with DOT. Those treated in this way had higher levels of treatment completion.19 In London, in 2003 only 12% of homeless patients started their treatment under direct observation but a further 47% were later switched to DOT after demonstrating poor adherence. Similar figures were seen for prisoners and problem drug users. This is despite national guidance in 199827 that “DOT is recommended for patients who are unlikely to comply” and, most recently, in 2006 the National Institute of Health and Clinical Excellence (NICE) recommended that “all patients should have a risk assessment for adherence to treatment, and DOT should be considered for patients who have adverse factors on their risk assessment; in particular street‐ or shelter‐dwelling homeless people and prisoners with active TB and patients with likely poor adherence and those who have a history of non‐adherence”.8
Greater emphasis is needed on practical measures to identify early patients at risk of poor adherence to treatment and to provide additional support including DOT from the start of treatment, access to appropriate accommodation, and use of incentives.28,29,30 DOT is unlikely to lead to improved treatment outcomes unless initiated in conjunction with a package of supportive care tailored to patients' needs.8,31,32
Most patients with TB pose a minimal transmission risk as they are smear negative on diagnosis, demonstrate good adherence to treatment and have high rates of treatment completion. By contrast, high levels of infectious and drug resistant disease, poor adherence and loss to follow‐up indicate that TB is not effectively controlled among homeless people, prisoners and problem drug users in London.
Acknowledgements
The authors thank all TB nurses, allied professionals and administrative staff in London who contributed data; Dr John Watson, Professor Anne Johnson and Dr Rob van Hest for comments on the manuscript; and the London Infectious Disease Research Network for support with ethical applications and analysis.
Abbreviations
DOT - directly observed therapy
TB - tuberculosis
Footnotes
Funding: Department of Health and the Health Protection Agency.
Competing interests: None.
Ethics approval: London Metropolitan Multi‐centre Research Ethics Committee
Statement of independence of researchers from funders: Alistair Story is currently employed by the Health Protection Agency
References
- 1.Health Protection Agency Focus on tuberculosis: annual surveillance report 2005 – England, Wales and Northern Ireland. London: Health Protection Agency Centre for Infections, 2006
- 2.Anon Isoniazid mono‐resistant tuberculosis in north London – update. CDR Weekly 200616(9) [Google Scholar]
- 3.Ruddy M C, Davies A P, Yates M D.et al Outbreak of isoniazid resistant tuberculosis in north London. Thorax 200459279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health Stopping tuberculosis in England: an action plan from the Chief Medical Officer. http://www.dh.gov.uk/PublicationsAndStatistics/Publications/PublicationsPolicyAndGuidance/PublicationsPolicyAndGuidanceArticle/fs/en?CONTENT_ID = 4090417&chk = DsgbSP London: Department of Health, 2004
- 5.Burman W J, Cohn D L, Rietmeijer C A.et al Noncompliance with directly observed therapy for tuberculosis. Epidemiology and effect on the outcome of treatment. Chest 19971111168–1173. [DOI] [PubMed] [Google Scholar]
- 6.Dye C, Watt C J, Bleed D M.et al Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA 20052932767–2775. [DOI] [PubMed] [Google Scholar]
- 7.Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev 200619(2)CD003343. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health, Clinical Excellence (NICE) Tuberculosis. Clinical diagnosis and management of tuberculosis and measures for its prevention and control. London: NICE, 2006, www.nice.org.uk/page.aspx?o = 296657 (accessed 5 Jan 2007)
- 9.Kumar D, Citron K M, Leese J.et al Tuberculosis among the homeless at a temporary shelter in London: a report of chest X‐ray screening programme. J Epidemiol Community Health 199549629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Southern A, Premaratne N, English M.et al Tuberculosis among homeless people in London: an effective model of screening and treatment. Int J Tuberc Lung Dis 199931001–1008. [PubMed] [Google Scholar]
- 11.Sycamore R. An Overview of Homelessness in London. Homeless Link 2002
- 12.Anon National statistics, 2001 Census information. London: Office for National Statistics, 2003, http://www.lho.org.uk/viewResource.aspx?id = 7923 (accessed 27 January 2006)
- 13.Anon Greater London Alcohol and Drug Alliance London: The highs and the lows. Greater London Authority, February 2003. http://www.london.gov.uk/mayor/health/drugs_and_alcohol/docs/highs‐lows‐exec‐sum.rtf (accessed 24 March 2006)
- 14.Anon Prison statistics England and Wales 2002. National Statistics. http://www.official‐documents.co.uk/document/cm59/5996/5996.pdf (accessed 27 January 2006)
- 15.Anon Annual report 2003. The state of the drugs problem in the European Union and Norway. European Monitoring Centre for Drugs and Drug Addiction, 2003. http://ar2003.emcdda.eu.int/en/home‐en.html (accessed 27 January 2006)
- 16.Office for National Statistics International migration – migrants entering or leaving England and Wales 2001. Series MN No.28. http://www.statistics.gov.uk/downloads/theme_population/MN28.pdf (accessed 27 January 2006)
- 17.Huber P J. The behaviour of maximum likelihood estimates under non‐standard conditions. In: Proceedings of the 5th Berkeley Symposium on Mathematical Statistics and Probability. Berkeley: University of California Press, 19671221–233. [Google Scholar]
- 18.White H. A heteroskedasticity‐consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 198048817–830. [Google Scholar]
- 19.Anon Maximum likelihood estimation of misspecified models. Econometrica 1982501–25. [Google Scholar]
- 20.Haddad M B, Wilson T W, Ijaz K.et al Tuberculosis and homelessness in the United States, 1994–2003. JAMA 20052932762–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss A R, Hahn J A, Tulsky J P.et al Tuberculosis in the homeless. A prospective study. Am J Respir Crit Care Med 2000162460–464. [DOI] [PubMed] [Google Scholar]
- 22.Salomon N, Perlman D C, Friedmann P.et al Prevalence and risk factors for positive tuberculin skin tests among active drug users at a syringe exchange program. Int J Tuberc Lung Dis 2000447–54. [PubMed] [Google Scholar]
- 23.Chaves F, Dronda F, Cave M D.et al A longitudinal study of transmission of tuberculosis in a large prison population. Am J Respir Crit Care Med 1997155719–725. [DOI] [PubMed] [Google Scholar]
- 24.de Vries G, van Hest R A. From contact investigation to tuberculosis screening of drug addicts and homeless persons in Rotterdam. Eur J Public Health 2005171101–1262. [DOI] [PubMed] [Google Scholar]
- 25.Anderson R M, May R M.Infectious diseases of humans: dynamics and control. Oxford: Oxford University Press, 1991
- 26.Golub J E, Mohan C I, Comstock G W.et al Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis 200591183–1203. [PMC free article] [PubMed] [Google Scholar]
- 27.Joint Tuberculosis Committee of the British Thoracic Society Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax 199853536–548. [PMC free article] [PubMed] [Google Scholar]
- 28.Pablos‐Mendez A, Knirsch C A, Barr R G.et al Nonadherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med 1997102164–170. [DOI] [PubMed] [Google Scholar]
- 29.LoBue P A, Cass R, Lobo D.et al Development of housing programs to aid in the treatment of tuberculosis in homeless individuals: a pilot study. Chest 1999115218–223. [DOI] [PubMed] [Google Scholar]
- 30.Bock N N, Sales R M, Rogers T.et al A spoonful of sugar …: improving adherence to tuberculosis treatment using financial incentives. Int J Tuberc Lung Dis 2001596–98. [PubMed] [Google Scholar]
- 31.Volmink J, Matchaba P, Garner P. Directly observed therapy and treatment adherence. Lancet 20003551345–1350. [DOI] [PubMed] [Google Scholar]
- 32.Sumartojo E. When tuberculosis treatment fails: a social behavioural account of patient adherence. Am Rev Respir Dis 19931471311–1320. [DOI] [PubMed] [Google Scholar]