Abstract
Background
Severe α1‐antitrypsin (AAT) deficiency is an autosomal recessive genetic condition associated with an increased but variable risk for chronic obstructive pulmonary disease (COPD). A study was undertaken to assess the impact of chronic bronchitis, pneumonia, asthma and sex on the development of COPD in individuals with severe AAT deficiency.
Methods
The AAT Genetic Modifier Study is a multicentre family‐based cohort study designed to study the genetic and epidemiological determinants of COPD in AAT deficiency. 378 individuals (age range 33–80 years), confirmed to be homozygous for the SERPINA1 Z mutation, were included in the analyses. The primary outcomes of interest were a quantitative outcome, forced expiratory volume in 1 s (FEV1) percentage predicted, and a qualitative outcome, severe airflow obstruction (FEV1 <50% predicted).
Results
In multivariate analysis of the overall cohort, cigarette smoking, sex, asthma, chronic bronchitis and pneumonia were risk factors for reduced FEV1 percentage predicted and severe airflow obstruction (p<0.01). Index cases had lower FEV1 values, higher smoking histories and more reports of adult asthma, pneumonia and asthma before age 16 than non‐index cases (p<0.01). Men had lower pre‐ and post‐bronchodilator FEV1 percentage predicted than women (p<0.0001); the lowest FEV1 values were observed in men reporting a history of childhood asthma (26.9%). This trend for more severe obstruction in men remained when index and non‐index groups were examined separately, with men representing the majority of non‐index individuals with airflow obstruction (71%). Chronic bronchitis (OR 3.8, CI 1.8 to 12.0) and a physician's report of asthma (OR 4.2, CI 1.4 to 13.1) were predictors of severe airflow obstruction in multivariate analysis of non‐index men but not women.
Conclusion
In individuals with severe AAT deficiency, sex, asthma, chronic bronchitis and pneumonia are risk factors for severe COPD, in addition to cigarette smoking. These results suggest that, in subjects severely deficient in AAT, men, individuals with symptoms of chronic bronchitis and/or a past diagnosis of asthma or pneumonia may benefit from closer monitoring and potentially earlier treatment.
Chronic obstructive pulmonary disease (COPD) is a complex disease characterised by variable susceptibility; this variability is probably determined by genes interacting with other predisposing host factors and environmental exposures. Homozygosity for the Z mutation in the SERPINA1 gene is an autosomal recessive genetic risk factor for COPD due to α1‐antitrypsin (AAT) deficiency. Although at high genetic risk, individuals with two severe deficiency alleles (eg, PI ZZ) have marked variability in the extent of COPD, consistent with the hypothesis that other factors may be relevant to the development of obstructive lung disease.
AAT is the main plasma inhibitor of neutrophil elastase, and the inherited deficiency of AAT has clearly been proved to be associated with the development of early onset emphysema.1 In the presence of AAT deficiency, cigarette smoking is an important contributor to accelerated decline in lung function, and the extent of lung injury is usually disproportionate to the amount smoked. The Z allele of the AAT gene is associated with a marked decrease in serum AAT levels, but studies of PI Z individuals have shown substantial variability of lung disease in smokers. In individuals who have never smoked cigarettes, there is also wide variability for the development of airflow obstruction.2,3 Factors such as asthma, pneumonia, other childhood respiratory illnesses,4,5,6 chronic bronchitis, differential impact of gender/sex biology and modifier genes all may have a role in the development of lung disease in individuals deficient in AAT.
The majority of individuals with severe AAT deficiency are identified after being diagnosed with lung or liver disease; based on gene frequency estimates, most PI ZZ subjects remain unidentified. The ascertainment scheme involved in identifying most PI ZZ subjects with existing liver or lung disease has biased insight into the natural history of PI ZZ individuals. As part of our AAT Genetic Modifier Study, we ascertained individuals based on the presence of two Z alleles (irrespective of lung/liver disease) and investigated characteristics that may be associated with obstructive lung disease in PI ZZ siblings and other relatives. Focusing on predictive characteristics in non‐index PI ZZ subjects partially avoids the issues of ascertainment bias and probably provides a more accurate view of the natural history of obstructive lung disease in PI ZZ individuals. However, since non‐index PI ZZ subjects are typically (but not always) relatives of individuals with lung and/or liver disease, the findings may not be completely generalisable to the entire PI ZZ population.
We have assembled a large cohort of PI ZZ index and non‐index subjects in families who have undergone standardised phenotyping with a questionnaire and spirometry. The careful phenotyping and inclusion of index and non‐index individuals provides a unique opportunity to test the hypothesis that ascertainment, sex, asthma, chronic bronchitis and pneumonia significantly and independently are associated with airflow obstruction in PI ZZ subjects.
Methods
Study sites and participants
The AAT Genetic Modifier Study is a multicentre study in collaboration with the Alpha‐1 Foundation, designed to study the genetic epidemiology of lung disease in PI ZZ individuals. The study protocol was reviewed by the individual Institutional Review Boards at each site.
Three hundred and seventy‐eight Caucasian PI ZZ subjects in 167 families were enrolled in the AAT Genetic Modifier Study. At least one PI ZZ sibling pair was necessary for a family to be eligible, with both siblings at least 30 years of age. All participants provided written informed consent. The study protocol included a questionnaire, spirometry and a blood sample for DNA and AAT studies.
Ascertainment scheme and proband designation
Ascertainment of eligible sibling pairs was based on confirmed homozygosity for the Z allele at the SERPINA1 locus (PI ZZ). The index case was the first person in the family diagnosed with AAT deficiency (irrespective of liver or lung disease), based on questionnaire responses. Each family only had one index case; all other ZZ family members were classified as non‐index. Index cases were not available for 27 families owing to death or non‐participation of some index cases.
Questionnaire
Each participant completed a computer‐based modified version of the ATS‐DLD Epidemiology Questionnaire.7 The specific questions for defining the presence or absence of chronic bronchitis, wheezing, asthma and pneumonia are summarised in the online supplement available at http://thorax.bmj.com/supplemental. Pack‐years of cigarette smoking were calculated by multiplying the number of years smoked by the average number of daily cigarettes smoked, divided by 20. “Ever smokers” answered yes to the question: “Have you ever smoked cigarettes?”; no was defined as <20 packs of cigarettes or 12 oz of tobacco in a lifetime or <1 cigarette a day for 1 year. “Current smokers” answered no to the question: “Have you stopped smoking (as of one month ago)?”
Spirometry
A full description of the spirometric methods is provided in the online supplement at http://thorax.bmj.com/supplemental. Pre‐ and post‐bronchodilator spirometry testing was performed according to American Thoracic Society standards8 with the Jaeger Masterscope PC Spirometer system (Jaeger, Hoechberg, Germany). For 16 subjects who had already undergone lung transplantation or lung volume reduction at the time of the study visit, spirometry that preceded the surgical procedure was obtained and the age used for analyses was the age at spirometry. Percentage predicted values were calculated using the predicted equations of Crapo and colleagues.9 Unless indicated, data are presented for pre‐bronchodilator forced expiratory volume in 1 s (FEV1). Bronchodilator reversibility was defined as the difference between absolute post‐ and pre‐bronchodilator FEV1 divided by baseline pre‐bronchodilator FEV1.
Confirmation of AAT phenotype
Blood spot cards for AAT studies were made from peripheral blood samples collected in EDTA. PI phenotyping was performed using the blood spot cards; specimens were assayed in polyacrylamide gels at pH 4.2–4.9 as previously described.10 The phenotype results were reported only if two independent interpretations agreed. Genotyping was performed using the Amplification and Refractory Mutation System (ARMS) technology for the detection of point mutations.11 A genotype of PI ZZ was assigned if the Z mutation was detected at position 342 and normal sequence was not detected.
Statistics
All computations were performed using the SAS statistical package (SAS Statistical Institute, Cary, North Carolina, USA) on a SUN Microsystem. χ2 and Student's t tests were used to compare counts and means, respectively. The Mann‐Whitney test was performed for non‐normally distributed covariates. Covariates considered for inclusion in regression models included binary variables (asthma (yes = 1, no = 0), sex (male = 1, female = 0), ever‐smoking status (yes = 1, no = 0), chronic bronchitis (yes = 1, no = 0), physician's diagnosis of asthma (yes = 1, no = 0), physician's diagnosis of pneumonia (yes = 1, no = 0), index case in family (yes = 1, no = 0)) and continuous variables (age, pack‐years of smoking). Multiple regression models were performed in SAS using the MIXED procedure in order to control for the correlation between family members.
Results
The 378 confirmed PI ZZ individuals represented 372 siblings, 2 PI ZZ parents, 1 PI ZZ uncle and 3 adult children of PI ZZ individuals. Individuals were ascertained for the study on the basis of the PI ZZ genotype, and all genotypes were confirmed. There were also 50 parents (47 MZ, 1 SZ, 2 missing PI type) enrolled in these families who were not included in the epidemiological analysis. Characteristics of the PI ZZ individuals in this cohort are provided in table 1.
Table 1 General demographic data of the 378 PI ZZ individuals*.
| Demographic characteristics | All (N = 378) | Index (N = 140) | Non‐index (N = 238) | p Value Index vs non‐index |
|---|---|---|---|---|
| Sex (female) | 205 (54%) | 73 (52%) | 132 (55%) | 0.53 |
| Mean (SD) age (years) | 52.2 (9.7) | 52.9 (9.5) | 51.7 (9.8) | 0.25 |
| Mean (SD) FEV1% predicted† | 65.9 (33.5) | 49.0 (28.5) | 72.3 (32.7) | <0.0001 |
| Mean (SD) FEV1/FVC ×100† | 55.1 (20.7) | 43.9 (17.3) | 61.4 (19.8) | <0.0001 |
| Smoking status | ||||
| Ever smoker | 233 (62%) | 105 (75%) | 128 (54%) | <0.0001 |
| Current smoker | 13 (3%) | 1 (<1%) | 12 (5%) | 0.03 |
| Median (IQR) pack‐years for smokers | 16 (7–24) | 17 (9–25) | 15 (6–22) | 0.02 |
| Median (IQR) age at onset of smoking (years) | 17 (15–19) | 17 (15–19) | 17 (15–19) | 0.70 |
| Lung trouble before age 16 | 93 (25%) | 41 (29%) | 52 (22%) | 0.11 |
| History of wheezing | 244(65%) | 102 (73%) | 142 (60%) | 0.01 |
| Doctor confirmed asthma | 139 (37%) | 64 (46%) | 75 (32%) | 0.006 |
| Asthma before age 16 | 20 (5%) | 13 (9%) | 7 (3%) | 0.008 |
| History of pneumonia | 220 (58%) | 95 (68%) | 125 (53%) | 0.004 |
| Pneumonia before age 16 | 47 (12%) | 17 (12%) | 30 (13%) | 0.90 |
| Chronic bronchitis | 83 (22%) | 33 (24%) | 50 (21%) | 0.56 |
FEV1, forced expiratory volume in 1 s.
*Includes 372 siblings, 2 parents, 1 uncle, 3 children of siblings.
†Post‐bronchodilator.
Although 62% of the cohort reported a history of cigarette smoking, only 3% were current smokers. The mean number of pack‐years of cigarette smoking was 18.2 for the smokers (median 17), and most started smoking during adolescence. Sixteen participants had undergone lung transplantation and/or lung volume reduction for the surgical management of emphysema. More than half of the participants reported a history of physician‐diagnosed pneumonia, 37% reported physician‐diagnosed asthma and 22% met the criteria for chronic bronchitis.
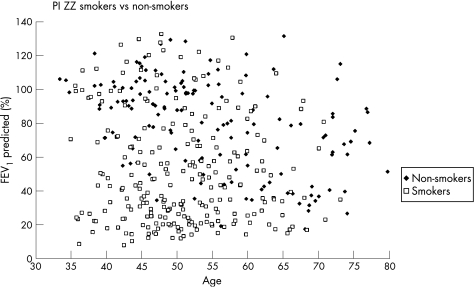
For all PI ZZ participants, FEV1 as a percentage of predicted was plotted as a function of age and stratified by smoking history (fig 1). As expected, the mean FEV1 was higher for non‐smokers than for smokers (non‐smokers vs smokers, p<0.0001). However, marked variability in FEV1 values was noted, with some life‐time non‐smokers having airflow obstruction before the age of 50. 61% of the cohort met the criteria for GOLD stage 2 or higher. We also examined the mean spirometric values and demographic features by index case designation (index or non‐index). There was no significant difference between the percentage of men and women classified as an index or non‐index subject (table 1). Index subjects had significantly lower spirometric measures and slightly higher pack‐years of cigarette smoking than non‐index subjects, and more non‐index subjects were current smokers. There was no difference in the reporting of general lung problems before the age of 16, but more index subjects reported a history of asthma before the age of 16. Although the number of individuals was small, a diagnosis of asthma before 16 years of age was a significant predictor of airflow obstruction (odds ratio (OR) 4.5 (95% CI 1.6 to 12.6, p = 0.004)), with 15 of the 20 individuals with asthma before age 16 having severe COPD. The mean (SD) FEV1 for subjects with asthma before age 16 was 39.8 (24.9)% predicted. More index subjects reported a history of physician‐diagnosed asthma and physician‐diagnosed pneumonia but not chronic bronchitis symptoms (table 1).
Figure 1 Forced expiratory volume in 1 s (FEV1) percentage predicted by age for 378 ZZ individuals stratified by smoking status. There is a wide variability in FEV1 for both smokers and non‐smokers, with some non‐smokers having low lung function and some smokers having preserved lung function.
Univariate and multivariate models were evaluated as predictors of FEV1 (percentage predicted) in all subjects, revealing that in addition to cigarette smoking and index case status, pneumonia, asthma, chronic bronchitis and male sex were associated with lower FEV1 (table 2). To assess the impact of using a different set of spirometric prediction equations, we repeated the analyses using the percentage predicted equations proposed by Hankinson et al.12 Using these equations, our results remained robust for the overall cohort and for the non‐index subgroup; the effect estimates for sex were attenuated only for the index case subgroup analysis.
Table 2 Univariable and multivariable predictors for forced expiratory volume in 1 s (FEV1, % predicted) in the overall cohort, index subjects and non‐index subjects.
| Overall | Index | Non‐index | |||||
|---|---|---|---|---|---|---|---|
| Beta (95% CI) | p Value | Beta (95% CI) | p Value | Beta (95% CI) | p Value | ||
| Univariable predictors | |||||||
| Sex | −19.69 (−13.17 to −26.21) | <0.0001 | −11.30 (−20.53 to −2.06) | 0.02 | −23.49 (−31.36 to−15.63) | <0.001 | |
| Pack‐years‡ | −1.10 (−0.13 to −0.89) | <0.0001 | −0.72 (−0.98 to −0.46) | <0.0001 | −1.19 (−1.49 to −0.89) | <0.0001 | |
| Ever smoking | −29.26 (−35.58 to −22.94) | <0.0001 | −25.31 (−35.32 to −15.30) | <0.0001 | −24.64 (−32.42 to −16.86) | <0.0001 | |
| Asthma | −19.08 (−25.87 to −12.30) | <0.0001 | −7.46 (−16.83 to 1.92) | 0.12 | −21.14 (−29.77 to −12.52) | <0.0001 | |
| Pneumonia | −21.25 (−27.70 to −14.70) | <0.0001 | −17.33 (−26.99 to −7.68) | 0.0005 | −18.09 (−26.15 to −10.02) | <0.0001 | |
| Chronic bronchitis | −17.264 (−25.28 to −9.25) | <0.0001 | −4.67 (−15.83 to 6.31) | 0.40 | −23.83 (−33.64 to −14.03) | <0.0001 | |
| Index | −26.34 (−32.74 to −19.73) | <0.0001 | – | – | – | – | |
| Multivariable predictors† | |||||||
| Sex | −15.51 (−20.58 to −10.43) | <0.0001 | −8.57 (−16.63 to −0.52) | 0.04 | −19.53 (−26.10 to −12.96) | <0.0001 | |
| Pack‐years‡ | −0.62 (−0.84 to −0.40) | <0.0001 | −0.51 (−0.80 to −0.22) | 0.0007 | −0.67 (−1.01 to −0.32) | 0.0002 | |
| Ever smoking | −10.36 (−16.86 to −3.85) | 0.002 | −13.33 (−24.34 to −2.33) | 0.02 | −8.62 (−17.04 to −0.20) | 0.04 | |
| Asthma | −12.47 (−17.79 to −7.15) | <0.0001 | −7.33 (−15.49 to 0.83) | 0.08 | −15.00 (−22.09 to −7.90) | <0.0001 | |
| Pneumonia | −14.38 (−19.51 to −9.25) | <0.0001 | −14.63 (−23.11 to −6.15) | 0.0009 | −14.49 (−20.98 to −8.00) | <0.0001 | |
| Chronic bronchitis | −9.26 (−15.35 to −3.16) | 0.003 | −5.24 (−14.70 to 4.22) | 0.28 | −11.34 (−19.43 to −3.26) | 0.006 | |
| Index | −15.19 (−20.54 to −9.84) | <0.0001 | – | – | |||
*Univariate model includes sex (male = 1, female = 0), pack‐years of cigarettes, ever‐smoking (yes = 1, no = 0), asthma (yes = 1, no = 0), pneumonia (yes = 1, no = 0), chronic bronchitis (yes = 1, no = 0), index case (yes = 1, no = 0)
†Multivariate model includes sex (male = 1, female = 0), pack‐years of cigarettes, ever‐smoking (yes = 1, no = 0), asthma (yes = 1, no = 0), pneumonia (yes = 1, no = 0), chronic bronchitis (yes = 1, no = 0), index case (yes = 1, no = 0).
‡Odds ratio estimate for pack‐years is for each pack‐year smoked.
We also evaluated bronchodilator reversibility in this cohort and observed that the mean bronchodilator responsiveness (measured as the percentage change in the absolute lung function as a function of the baseline FEV1) was 6.7%. As expected, bronchodilator responsiveness was associated with lower FEV1 (p<0.0001). In univariate models, significant predictors of bronchodilator responsiveness included pneumonia (p = 0.01), male sex (p = 0.03) and pack‐years of smoking (p = 0.0008); there was a trend for physician‐diagnosed asthma (p = 0.06). In a multivariate model that included sex, pack‐years of smoking, asthma and pneumonia, only sex, pack‐years of smoking and pneumonia remained significant predictors (p<0.05).
Logistic regression analysis was performed to identify predictors of severe airflow obstruction in all subjects (table 3); these models for severe COPD showed the same significant predictors as FEV1 analysed as a quantitative outcome.
Table 3 Univariable and multivariable models for predicting severe airflow obstruction in the overall cohort, index subject and non‐index subjects.
| All OR (95% CI) | Index OR (95% CI) | Non‐index OR (95% CI) | |
|---|---|---|---|
| Number with severe airflow obstruction | 158/377 (42%) | 90/140 (64%) | 68/237 (27%) |
| Univariable predictors* | |||
| Age | 1.01 (0.99 to 1.03) | 1.07 (0.97 to 1.05) | 1.01 (0.98 to 1.04) |
| Sex | 3.14 (2.05 to 4.80) | 2.43 (1.18 to 4.97) | 4.59 (2.49 to 8.46) |
| Pack‐years of cigarettes‡ | 1.09 (1.06 to 1.11) | 1.09 (1.05 to 1.13) | 1.08 (1.05 to 1.10) |
| Ever smoking status | 6.89 (4.14 to 11.47) | 5.54 (2.43 to 12.62) | 6.44 (3.47 to 12.90) |
| History of asthma | 3.14 (2.03 to 4.85) | 2.43 (1.18 to 5.01) | 3.33 (1.78 to 5.84) |
| History of pneumonia | 3.57 (2.28 to 5.58) | 3.50 (1.66 to 7.37) | 3.16 (1.71 to 5.81) |
| Chronic bronchitis | 2.98 (1.80 to 4.93) | 2.02 (0.83 to 4.90) | 4.18 (2.17 to 8.05) |
| Index case | 4.47 (2.86 to 6.99) | – | – |
| Multivariable predictors† | |||
| Sex | 4.05 (2.63 to 7.45) | 2.64 (1.05 to 6.53) | 5.51 (2.57 to 11.81) |
| Pack‐years of cigarettes‡ | 1.06 (1.03 to 1.09) | 1.10 (1.04 to 1.16) | 1.04 (1.00 to 1.08) |
| Ever smoking status | 2.75 (1.27 to 5.97) | 1.51 (0.42 to 5.43) | 3.66 (1.36 to 9.87) |
| History of asthma | 3.24 (1.81 to 5.81) | 3.70 (1.45 to 9.44) | 3.18 (1.47 to 6.89) |
| History of pneumonia | 4.09 (2.25 to 7.45) | 4.71 (1.80 to 12.33) | 3.89 (1.78 to 8.50) |
| Chronic bronchitis | 2.63 (1.37 to 5.07) | 2.77 (0.90 to 8.47) | 2.78 (1.22 to 6.35) |
| Index case | 3.53 (1.99 to 6.24) | – | – |
*Univariate model includes sex (male = 1, female = 0), pack‐years of cigarettes, ever‐smoking (yes = 1, no = 0), asthma (yes = 1, no = 0), pneumonia (yes = 1, no = 0), chronic bronchitis (yes = 1, no = 0), index case (yes = 1, no = 0).
†Multivariate model includes sex (male = 1, female = 0), pack‐years of cigarettes, ever‐smoking (yes = 1, no = 0), asthma (yes = 1, no = 0), pneumonia (yes = 1, no = 0), chronic bronchitis (yes = 1, no = 0), index case (yes = 1, no = 0).
‡Estimate for pack‐years is for each pack‐year smoked.
The odds for severe airflow obstruction in the overall cohort were highest for index cases, men, individuals with a history of cigarette smoking and subjects with a history of asthma, chronic bronchitis and/or pneumonia.
Because being the index case in a family was a strong predictor of reduced FEV1 and severe airflow obstruction, we parsed the group by index case status (index versus non‐index) and performed the same covariate modelling in the stratified cohort (tables 2 and 3). In multivariate analysis, smoking, sex, a history of asthma and a history of pneumonia were predictors of severe airflow obstruction in both index and non‐index individuals, but chronic bronchitis was a significant predictor of airflow obstruction only in non‐index individuals (table 3). Similar results were obtained for FEV1 as a quantitative outcome (table 2), although asthma was not significantly associated in index cases (p = 0.08).
We subsequently investigated the characteristics of the 378 PI ZZ individuals stratified by sex, as sex was a highly significant predictor of both FEV1 and severe airflow obstruction in univariate and multivariate models. The overall demographic characteristics for men and women are presented in table 4 (and in supplemental tables 1 and 2 available online at http://thorax.bmj.com/supplemental).
Table 4 Sex‐specific demographic and clinical characteristics in PI ZZ subjects.
| Demographic characteristic | Men (N = 173) | Women (N = 205) | p Value |
|---|---|---|---|
| Age | 52.0 (9.1) | 52.4 (10.2) | 0.69 |
| FEV1 % predicted (post‐bronchodilator) | 55.4 (32.2) | 74.9 (31.9) | <0.0001 |
| FEV1/FVC ×100 (post‐bronchodilator) | 48.2 (20.6) | 61.1 (18.9) | <0.0001 |
| Smoking status | |||
| Ever smoker (n) | 114 | 119 | 0.12 |
| Pack‐years (smokers) | 20.9 (14.7) | 15.6 (13.9) | 0.005 |
| Age at onset of smoking | 17.0 (3.4) | 18.6 (5.7) | 0.008 |
Values are mean (SD) unless otherwise indicated.
FEV1, forced expiratory volume in 1 s.
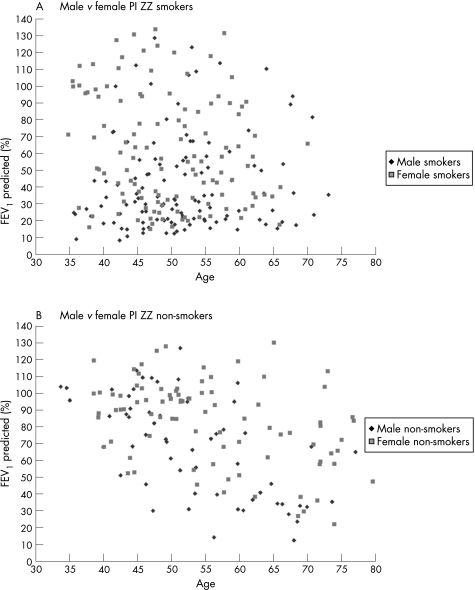
Although there was no difference in the percentage of men and women who reported a history of cigarette smoking, on average men smoked a mean of 5.3 pack‐years more and started smoking at a younger age. Overall, men had significantly lower values for FEV1 (percentage predicted) and FEV1/FVC (table 4); this trend persisted even among non‐smokers, with men who were non‐smokers having lower pre‐ and post‐bronchodilator spirometric measures than women (pre‐bronchodilator FEV1: 72.2% predicted in men vs 86.3% predicted in women (p = 0.003); pre‐bronchodilator FEV1/FVC: 58.7 in men vs 69.2 in women (p = 0.0006), with similar results for post‐bronchodilator spirometry with p⩽0.002). For all PI ZZ men and women, FEV1 (percentage predicted) was plotted separately for smokers and non‐smokers as a function of age and stratified by sex (fig 2A and B), further demonstrating this marked variation in FEV1 for women and men. We repeated the analyses using the percentage predicted equations proposed by Hankinson et al.12 Our results remained robust regardless of the predicted models used. There were no differences between the percentages of men and women who reported “lung problems before age 16”, wheezing, physician diagnosis of asthma or pneumonia, although there was a significant trend for men to report chronic bronchitis more frequently (p = 0.05) (data not shown). The most marked contrast for mean FEV1 was for those individuals reporting a history of asthma before age 16 (men 26.9% predicted, women 55.0% predicted, p = 0.03). Despite a similar sex distribution of men and women in the index and non‐index categories, female index and non‐index subjects had higher FEV1 and FEV1/FVC values than men.
Figure 2 Percentage predicted forced expiratory volume in 1 s (FEV1) by age for (A) ZZ smokers and (B) ZZ non‐smokers stratified by sex. Male smokers tend to have lower FEV1 than female smokers (p<0.0001) and male non‐smokers tend to have lower FEV1 than female non‐smokers (p = 0.003).
For the qualitative analysis of severe airflow obstruction we focused on the non‐index subjects. Only 15% of the female non‐index subjects had severe airflow obstruction. The predictors of severe airflow obstruction were the same for male and female non‐index subjects on univariate analysis and, on multivariate analysis, asthma and chronic bronchitis remained robust predictors of airflow obstruction only in men (table 5).
Table 5 Univariable and multivariable models for predicting severe airflow obstruction in the 237 PI ZZ non‐index men and women.
| Men OR (95% CI) | Women OR (95% CI) | |
|---|---|---|
| Number with severe airflow obstruction | 48/106 | 20/131 |
| Univariable predictors* | ||
| Pack‐years of cigarettes‡ | 1.07 (1.04 to 1.11) | 1.07 (1.02 to 1.11) |
| Ever smoking status | 6.14 (2.51 to 15.00) | 7.72 (2.14 to 27.86) |
| History of asthma | 6.70 (2.54 to 17.72) | 2.89(1.10 to 7.62) |
| History of chronic bronchitis | 5.20 (1.96 to 13.82) | 3.20 (1.11 to 9.23) |
| History of pneumonia | 4.61 (2.02 to 10.53) | 2.95 (1.00 to 8.66) |
| Multivariable predictors† | ||
| Pack‐years of cigarettes‡ | 1.05 (1.00 to 1.10) | 1.03 (0.98 to 1.08) |
| Ever smoking status | 2.64 (0.65 to 10.68) | 5.41 (1.20 to 24.41) |
| History of asthma | 4.24 (1.38 to 13.07) | 2.44 (0.81 to 7.33) |
| History of chronic bronchitis | 3.76 (1.18 to 11.97) | 1.95 (0.56 to 6.84) |
| History of pneumonia | 4.14 (1.49 to 11.54) | 3.51 (1.04 to 11.85) |
*Univariate model includes sex (male = 1), pack‐years of cigarettes, ever‐smoking (yes = 1), asthma (yes = 1), pneumonia (yes = 1), chronic bronchitis (yes = 1), index case (yes = 1).
†Multivariate model includes sex (male = 1), pack‐years of cigarettes, ever‐smoking (yes = 1), asthma (yes = 1), pneumonia (yes = 1), chronic bronchitis (yes = 1), index case (yes = 1).
‡Estimate for pack‐years is for each pack‐year smoked.
Discussion
The development of COPD in the setting of severe AAT deficiency is highly variable. Host factors such as modifier genes probably interact with environmental factors to contribute to an individual's manifestations of lung disease. Many people are tested for AAT deficiency due to existing lung disease, and this ascertainment scheme biases observations of the natural history of lung function. Previous studies have raised concerns about the influence of ascertainment bias in studies designed to investigate determinants of lung disease in PI ZZ subjects. One important and unique feature of our cohort is that it represents the first large collection of sibling pairs and family members systematically enrolled on the basis of homozygosity for the Z mutation of the SERPINA1 gene, irrespective of the presence of lung or liver disease. Evaluating the predictors of airflow obstruction in the non‐index individuals in such families may provide insight into predictors of variable severity of COPD in AAT deficient individuals.
Our study suggests that asthma, pneumonia, chronic bronchitis and sex have the largest impact on non‐index subjects with AAT deficiency, many of whom still have lung function in the normal range; asthma and chronic bronchitis are not significant predictors in index individuals. When we focused on identifying risk factors for severe COPD, sex, smoking, pneumonia and chronic bronchitis all had odds ratios >2, and these predictors could identify important pathways for genetic modifiers of COPD in AAT deficiency. A childhood history of asthma, although present in a small number of individuals in our study, was associated with the most severe COPD. Although the individual predictors that we investigated have been suggested as predictors of lung function in some previous studies of AAT deficient individuals, this observed difference in families for predictors of lung function between index and non‐index individuals, as well as men and women, provides insight into the variable susceptibility to COPD in AAT deficiency as well as familial discordance for COPD, despite similar smoking histories.
Cigarette smoking is the most important risk factor for the development of emphysema in AAT deficient individuals, but the variability and dose‐response to this exposure suggest the importance of genetic and other environmental factors. Similar to other investigators,13,14,15,16,17,18 we have observed that smoking was often but not always associated with lower FEV1. Individuals with AAT deficiency who smoke often develop obstructive lung disease at an early age,13,14,16 but we have corroborated that some current and former smokers have preserved lung function.4 We also observed that sex, smoking and a history of pneumonia were important predictors of lung function in index and non‐index individuals. Chronic bronchitis and a physician diagnosis of asthma contributed to the severity of COPD in non‐index individuals. This observation in non‐index individuals, who tend to have higher FEV1, provides an insight into the types of symptoms (cough, sputum and wheeze) that may portend susceptibility to lung function decline and COPD among ZZ individuals. Further research will be required to determine whether the increased risk of airflow obstruction in individuals with symptoms of chronic cough and phlegm and episodes of pneumonia could relate to bronchiectasis, which is also associated with AAT deficiency.19
Airway hyperresponsiveness and wheezing may be susceptibility phenotypes for worse lung outcomes, especially in the setting of cigarette smoking. In this cohort, 25% had at least a 10% increase in FEV1 and this reversibility was associated with lower lung function. Although emphysema/COPD may be misdiagnosed as asthma in adults with AAT deficiency,20 a diagnosis of asthma in childhood is unlikely to be due to emphysema/COPD. In individuals in Sweden identified with AAT deficiency as part of neonatal screening, 15% were given a diagnosis of asthma by age 22 and 29% self‐reported recurrent wheezing episodes.21 We observed that a physician diagnosis of asthma before age 16 was strongly associated with reduced FEV1 and severe COPD in adulthood, suggesting asthma (or the presence of asthma‐like symptoms) may define a particularly susceptible subset of PI ZZ individuals. In our study the most profound reduction in lung function was among men with a history of asthma before age 16, and a physician diagnosis of asthma remained an important predictor of airflow obstruction in non‐index men (but not women), which suggests that one potential difference between men and women with COPD may be related to lung inflammation. Inflammation associated with asthma may portend the development of airflow obstruction in later life through limiting the level of maximally achieved lung function or through accelerating lung function decline. Previous studies have shown an association between asthmatic features and COPD in adults with AAT deficiency,5,6,22 and asthmatic symptoms in adults have previously been suggested as a risk factor for lower FEV1.3,18
Pneumonia was associated with lower FEV1 and severe airflow obstruction in individuals with AAT deficiency in our study cohort, although it is unclear if pneumonia precedes or follows COPD. Although pneumonia has been suggested as a risk factor for COPD in the past,4 this has not been a consistent observation.3,15 Interestingly, we observed that, although a physician diagnosis of pneumonia was associated with lower lung function in both men and women, a report of pneumonia before age 16 was not, suggesting that pneumonia may be a consequence rather than a cause of COPD.
Sex has been reported as a risk factor for lower FEV1 in some but not all previous studies of AAT deficient individuals. An increased risk for lung disease in men has been noted by various investigators.3,13,14 No significant effect was observed by Silverman and colleagues,4 but the small sample size in that study may have limited the ability to detect a gender effect. The higher number of men among identified PI Z individuals has been attributed to higher rates of smoking among men,18,23 but our current data and previous data in non‐smokers3 suggest that there are sex‐based differences for susceptibility to lung disease in AAT deficiency in addition to cigarette smoking. Men had lower spirometric measurements than women in our cohort, regardless of index case or smoking status, age and/or a history of pneumonia, chronic bronchitis or asthma. Larsson and colleagues14 noted a low prevalence of COPD in non‐smoking women in their cohort. Tobin and colleagues13 observed wide variability in lung function among PI Z non‐smokers but also noted that older female non‐smokers had less limitation of lung function than older males. They also noted that FEV1 percentage predicted was lower in men than women and that the effects of sex and smoking on multiple regression analysis were significant, although there was no significance of a sex‐by‐smoking interaction term.13 Although we, like Tobin and colleagues, did not observe a significant interaction term for smoking and sex (results not shown), it may be more likely that the significant interaction will be modifier gene‐by‐sex factors. Other environmental exposures, such as occupational dust exposures, are also important to consider as potential modifiers of the risk for COPD in AAT deficiency. Piitulainen and colleagues3 observed that male non‐smokers were at increased risk for low lung function compared with female non‐smokers. In a sex‐stratified analysis they observed that age was an independent predictor of FEV1 in both men and women but “wheeziness” was a predictor only in men. In our study, men had lower spirometric measures than women and, among the non‐index cases, symptoms of chronic bronchitis and a physician diagnosis of asthma were significant predictors of severe airflow obstruction only for men. Although this may be because of the lower number of non‐index women with airflow obstruction, as noted above, men with AAT deficiency may have increased inflammation associated with asthma and chronic bronchitis symptoms.
There have been many previous studies of risk factors for reduced lung function in PI ZZ individuals, but our study has several unique features. First, only subjects with a confirmed PI ZZ genotype were considered; second, we used standardised spirometry with the same spirometry system across centres to minimise technical variability from the spirometric measures; third, we collected a large number of non‐index cases and adjusted for potential familial correlations within the multivariate models. Limitations include a potential effect of recall bias for childhood asthma as well as physician's diagnosis of pneumonia and asthma. There is also the potential for misclassification bias associated with COPD diagnosed as asthma. We do not have corroborating data such as chest radiological studies, sputum assessments for pneumonia or methacholine testing for asthma. Ascertainment bias is also important to consider as a limitation, although focusing on the observations in the non‐index subjects partially broaches this bias. Although a complete understanding of the natural history of lung disease in PI ZZ individuals will require enrolling subjects without ascertainment bias from a large general population sample, our current study confirms that (in addition to cigarette smoking) sex, asthma, chronic bronchitis and possibly pneumonia are risk factors for reduced FEV1 and severe airflow obstruction in PI ZZ individuals. These results suggest that, among those with severe AAT deficiency, men, individuals with symptoms of chronic bronchitis and/or a past diagnosis of asthma or pneumonia may benefit from closer monitoring and potentially earlier treatment.
Genetic modifiers are likely to be important in the variable development of COPD in individuals with severe AAT deficiency. We have identified several determinants of COPD in a large collection of siblings homozygous at the PI ZZ risk locus. Using family‐based genetic methods, these epidemiological factors may identify important genetic modifiers (such as asthma genetic modifiers of COPD in PI ZZ individuals) and key interactions (such as gene‐by‐sex) that will provide new insights into the pathophysiology and natural history of lung disease in PI ZZ individuals with AAT deficiency.
Further details are given in the online supplement available at http://thorax.bmj.com/supplemental.
Acknowledgements
The authors thank all the participants in the AAT Genetic Modifier Study for their enthusiastic support and participation and Dr Robert Crapo for his review and quality assurance of a subset of the spirometry data.
Abbreviations
AAT - α1‐antitrypsin
COPD - chronic obstructive pulmonary disease
FEV1 - forced expiratory volume in 1 s
FVC - forced vital capacity
Footnotes
Funding: K08 HL072918 (DLD), ALA Research Grant (DLD), R01 HL68926 (EKS) and an ALA Career Investigator Award (EKS).
Competing interests: None.
Further details are given in the online supplement available at http://thorax.bmj.com/supplemental.
References
- 1.Laurell C B, Eriksson S. The electrophoretic alpha 1‐globulin pattern of serum in alpha 1‐antitrypsin deficiency. Scand J Clin Lab Invest 196315132–140. [DOI] [PubMed] [Google Scholar]
- 2.Black L F, Kueppers F. Alpha‐1‐antitrypsin deficiency in nonsmokers. Am Rev Respir Dis 1978117421–428. [DOI] [PubMed] [Google Scholar]
- 3.Piitulainen E, Tornling G, Eriksson S. Effect of age and occupational exposure to airway irritants on lung function in non‐smoking individuals with alpha 1‐antitrypsin deficiency (PiZZ). Thorax 199752244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman E K, Pierce J A, Province M A.et al Variability of pulmonary function in alpha‐1‐antitrypsin deficiency: clinical correlates. Ann Intern Med 1989111982–991. [DOI] [PubMed] [Google Scholar]
- 5.Eden E, Hammel J, Rouhani F N.et al Asthma features in severe alpha1‐antitrypsin deficiency: experience of the National Heart, Lung, and Blood Institute Registry. Chest 2003123765–771. [DOI] [PubMed] [Google Scholar]
- 6.Eden E, Mitchell D, Mehlman B.et al Atopy, asthma, and emphysema in patients with severe alpha‐1‐antitrypysin deficiency. Am J Respir Crit Care Med 199715668–74. [DOI] [PubMed] [Google Scholar]
- 7.Ferris B G. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 19781181–120. [PubMed] [Google Scholar]
- 8.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 19951521107–1136. [DOI] [PubMed] [Google Scholar]
- 9.Crapo R O, Morris A H, Gardner R M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981123659–664. [DOI] [PubMed] [Google Scholar]
- 10.Pierce J A, Eradio B G. Improved identification of antitrypsin phenotypes through isoelectric focusing with dithioerythritol. J Lab Clin Med 197994826–831. [PubMed] [Google Scholar]
- 11.Stockley R A, Campbell E J. Alpha‐1‐antitrypsin genotyping with mouthwash specimens. Eur Respir J 200117356–359. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson J L, Odencrantz J R, Fedan K B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999159179–187. [DOI] [PubMed] [Google Scholar]
- 13.Tobin M J, Cook P J, Hutchison D C. Alpha 1 antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in subjects homozygous for Pi type Z. A survey by the British Thoracic Association. Br J Dis Chest 19837714–27. [DOI] [PubMed] [Google Scholar]
- 14.Larsson C. Natural history and life expectancy in severe alpha1‐antitrypsin deficiency, Pi Z. Acta Med Scand 1978204345–351. [DOI] [PubMed] [Google Scholar]
- 15. Alpha‐1‐Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha‐1‐antitrypsin. Am J Respir Crit Care Med 199815849–59. [DOI] [PubMed] [Google Scholar]
- 16.Janus E D, Phillips N T, Carrell R W. Smoking, lung function, and alpha 1‐antitrypsin deficiency. Lancet 19851152–154. [DOI] [PubMed] [Google Scholar]
- 17.Seersholm N, Kok‐Jensen A, Dirksen A. Decline in FEV1 among patients with severe hereditary alpha 1‐antitrypsin deficiency type PiZ. Am J Respir Crit Care Med 19951521922–1925. [DOI] [PubMed] [Google Scholar]
- 18.Silverman E K, Miletich J P, Pierce J A.et al Alpha‐1‐antitrypsin deficiency. High prevalence in the St. Louis area determined by direct population screening. Am Rev Respir Dis 1989140961–966. [DOI] [PubMed] [Google Scholar]
- 19.Dowson L J, Guest P J, Stockley R A. The relationship of chronic sputum expectoration to physiologic, radiologic, and health status characteristics in alpha(1)‐antitrypsin deficiency (PiZ). Chest 20021221247–1255. [DOI] [PubMed] [Google Scholar]
- 20.Stoller J K, Smith P, Yang P.et al Physical and social impact of alpha 1‐antitrypsin deficiency: results of a survey. Cleve Clin J Med 199461461–467. [DOI] [PubMed] [Google Scholar]
- 21.Piitulainen E, Sveger T. Respiratory symptoms and lung function in young adults with severe alpha(1)‐antitrypsin deficiency (PiZZ). Thorax 200257705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eden E, Strange C, Holladay B.et al Asthma and allergy in alpha‐1 antitrypsin deficiency. Respir Med 20061001384–1391. [DOI] [PubMed] [Google Scholar]
- 23.Brantly M L, Paul L D, Miller B H.et al Clinical features and history of the destructive lung disease associated with alpha‐1‐antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis 1988138327–336. [DOI] [PubMed] [Google Scholar]