Abstract
Coenzyme Q10 is an essential cofactor of the electron transport chain as well as a potent free radical scavenger in lipid and mitochondrial membranes. Feeding with coenzyme Q10 increased cerebral cortex concentrations in 12- and 24-month-old rats. In 12-month-old rats administration of coenzyme Q10 resulted in significant increases in cerebral cortex mitochondrial concentrations of coenzyme Q10. Oral administration of coenzyme Q10 markedly attenuated striatal lesions produced by systemic administration of 3-nitropropionic acid and significantly increased life span in a transgenic mouse model of familial amyotrophic lateral sclerosis. These results show that oral administration of coenzyme Q10 increases both brain and brain mitochondrial concentrations. They provide further evidence that coenzyme Q10 can exert neuroprotective effects that might be useful in the treatment of neurodegenerative diseases.
Coenzyme Q is an essential cofactor in the electron transport chain where it accepts electrons from complex I and II (1–3). Coenzyme Q also serves as an important antioxidant in both mitochondria and lipid membranes (4, 5). Coenzyme Q, which also is known as ubiquinone, is a lipid-soluble compound composed of a redox active quinoid moiety and a hydrophobic “tail.” The predominant form of coenzyme Q in humans is coenzyme Q10, which contains 10 isoprenoid units in the tail, whereas the predominant form in rodents is coenzyme Q9, which has nine isoprenoid units in the tail. Coenzyme Q is soluble and mobile in the hydrophobic core of the phospholipid bilayer of the inner membrane of the mitochondria where it transfers electrons one at a time to complex III of the electron transport chain.
There has been considerable interest in the use of coenzyme Q10 for the treatment of mitochondrial disorders. Several reports found both clinical and biochemical improvement in patients with mitochondrial disorders (6–10). If defects in energy metabolism and oxidative damage play a role in the pathogenesis of neurodegenerative diseases (11, 12), then treatment with coenzyme Q10 could exert beneficial therapeutic effects. We previously showed that oral administration of coenzyme Q10 significantly attenuated lesions produced by intrastriatal administration of malonate in rats, as well as malonate-induced depletions of ATP and increases in lactate concentrations (13). In the present study, we examined the effects of oral administration of coenzyme Q10 on brain and brain mitochondrial concentrations. We examined both oxidized and reduced coenzyme Q10 levels because the latter is the form that exerts antioxidant effects (4, 5). We examined neuroprotective effects against striatal lesions produced by systemic administration of 3-nitropropionic acid (3-NP) and survival in a transgenic animal model of familial amyotrophic lateral sclerosis (ALS).
MATERIALS AND METHODS
Studies of coenzyme Q10 were carried out in male Sprague–Dawley rats. Coenzyme Q10 powder (Vitaline Formulas, Ashland, OR) was formulated in rat chow (Agway Prolab 3200, Syracuse, NY). Animals were treated at a dose of 200 mg/kg per day, a dose that we previously found was neuroprotective (13). Controls received unsupplemented rat chow. We examined the effects of oral administration of coenzyme Q10 at 200 mg/kg for up to 2 months in 12-month-old male Sprague–Dawley rats as compared with 12-month-old animals on unsupplemented rat chow (n = 7). Animals were sacrificed, and cerebral cortex was dissected and frozen at −80°C. Mitochondrial preparations were made as previously described (14). In a follow-up experiment we examined the effects of oral administration of coenzyme Q10 at a dose of 200 mg/kg for 1 month in 24-month-old Fisher 344 rats as compared with unsupplemented rat chow (n = 8).
Coenzyme Q10 and vitamin E measurements were made by HPLC with electrochemical detection. Tissue samples weighing 50 mg were sonicated in 0.4 ml of HPLC running buffer consisting of 50% methanol/50% ethanol with 0.1 M sodium perchlorate, 6.15 mM perchloric acid. The samples were centrifuged at 12,000 g twice for 10 min. Twenty microliters of the supernatant was injected. The system consisted of WISP 12B refrigerated autosampler, a Waters 510 HPLC pump, a Nikko Bioscience 15 cm 3 μm C18 column (ESA, Bedford, MA), and an ESA model 5100A coulochem detector. Samples are eluted isocratically with the running buffer at 1 ml/min. The guard cell and the first electrochemical cell were set at −600 mV whereas the second cell was set at +200 mV. Coenzyme Q9, coenzyme Q10, and α-tocopherol standards were obtained from Sigma. Reduced standards were made by reacting coenzyme Q9 or coenzyme Q10 with dithionite and extracting in hexane. The retention times of standards were: α tocopherol, 4.9 min; coenzyme Q9 H2, 8 min; coenzyme Q10 H2, 10 min; coenzyme Q9, 12 min, and coenzyme Q10, 20 min. The sensitivity of the assay for coenzyme Q10 at a signal to noise of 5:1 is 0.5 ng. Recovery of spiked samples is 93.3 ± 8.4% (mean ± SD) n = 4. The identity of peaks was confirmed by coelution with synthetic standards and by varying chromatographic conditions. We also verified peaks in samples by reducing with dithionite and showing that the oxidized coenzyme Q9 and Q10 peaks disappeared with a corresponding increase in reduced coenzyme Q9 and Q10 peaks.
The effects of oral administration of coenzyme Q10 on 3-NP-induced striatal lesions were examined in 300- to 350-g rats (n = 10). Animals received either rat chow supplemented with coenzyme Q10 at 200 mg/kg or unsupplemented rat chow. After 1 week they were treated with 3-NP at a dose of 10 mg/kg i.p. twice a day until either a control or treated animal became symptomatic with hindlimb dystonia, and then sacrificed in pairs as previously described (15). Animals became ill after 5–6 days. Brains were removed, and 2-mm thick sections were stained with 2% 2,3,5-triphenyltetrazolium chloride (in the dark, at room temperature, 30 min), and then placed in 4% paraformaldehyde, pH 7.3. Lesions, demarcated by pale staining, were evaluated on the posterior surface of each section by using a Bioquant 4 system, by an experienced histologist blinded to experimental conditions. In the same rats we measured reduced and oxidized coenzyme Q9 and Q10 levels in a section of frontal cortex.
To determine whether coenzyme Q10 exerts neuroprotective effects in a transgenic mouse model of a human neurodegenerative disorder, we administered coenzyme Q10 (200 mg/kg) orally to 16 transgenic mice overexpressing a human Cu/Zn superoxide dismutase (SOD1) mutation, as compared with 13 animals that received unsupplemented rat chow. The mice used were the G1 line, which expresses high levels of human SOD with the G93A mutation (16). Treatment was started at 50 days after birth. We were unable to obtain reliable data for disease onset as assessed behaviorally. We therefore examined the time to mortality as a primary end point. Treatment was continued until mice reached end-stage disease. At end stage, mice laid on their sides in their cage and were fed moistened food. They were sacrificed if they could not right themselves from a flat surface within 30 sec or if they could not groom their faces.
The data are expressed as the means ± SEM. Statistical comparisons were made by unpaired Student’s t test or one-way ANOVA with the Fisher protected least significance difference (PLSD) test. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local animal care committee.
RESULTS
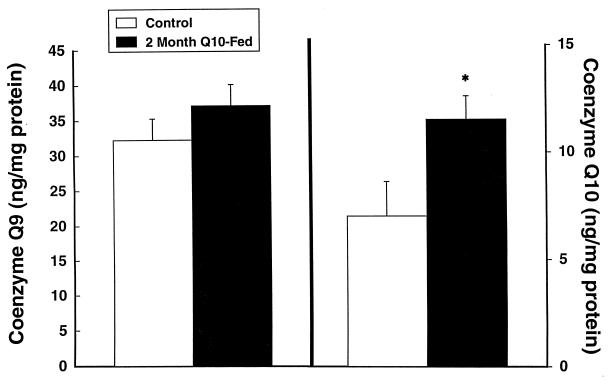
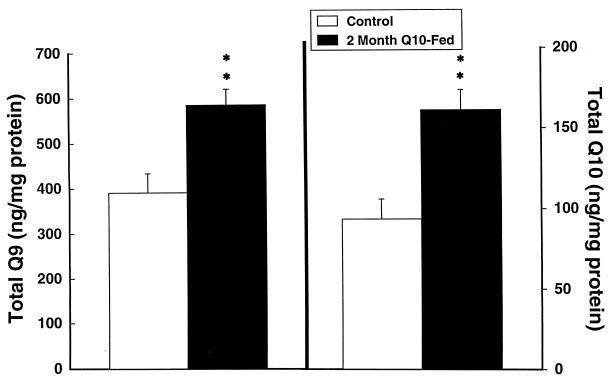
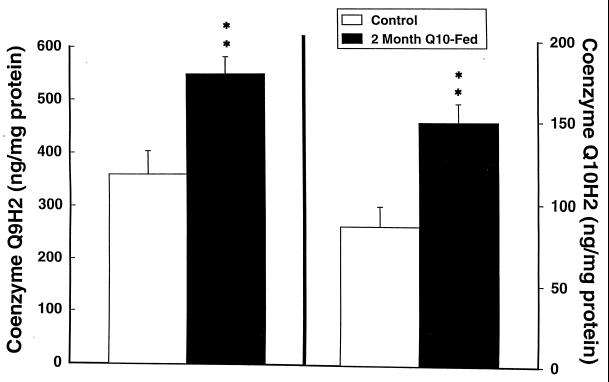
Coenzyme Q levels are known to decrease with aging in several animal species and in humans (17, 18). We therefore administered coenzyme Q10 to 12- and 24-month-old rats. Oral supplementation with coenzyme Q10 at a dose of 200 mg/kg to 12-month-old Sprague–Dawley rats produced significant increases in coenzyme Q10, coenzyme Q9 H2, coenzyme Q10 H2, and total coenzyme Q9 and coenzyme Q10 levels in cerebral cortex (Figs. 1–3). Increases were in the 30% range. The increases restored levels of coenzyme Q9 and coenzyme Q10 to levels similar to those seen in young animals (2–3 months old). In 24-month-old Fisher 344 rats fed with coenzyme Q10 for 1 month there was a significant increase in cerebral cortex-reduced coenzyme Q10 from 68.6 ± 3.9 to 74.4 ± 2.8 ng/mg of protein (P < 0.05), and the total coenzyme Q10 concentration increased from 75.2 ± 4.0 to 81.4 ± 2.7 ng/mg of protein (P < 0.05).
Figure 1.
Cerebral cortex concentrations of oxidized coenzyme Q9 and coenzyme Q10 in 12-month-old rats treated for 2 months with 200 mg/kg of coenzyme Q10. ∗, P < 0.05.
Figure 3.
Cerebral cortex concentrations of total coenzyme Q9 (oxidized and reduced) and total coenzyme Q10 in 12-month-old rats treated with 200 mg/kg of coenzyme Q10. ∗∗, P < 0.01.
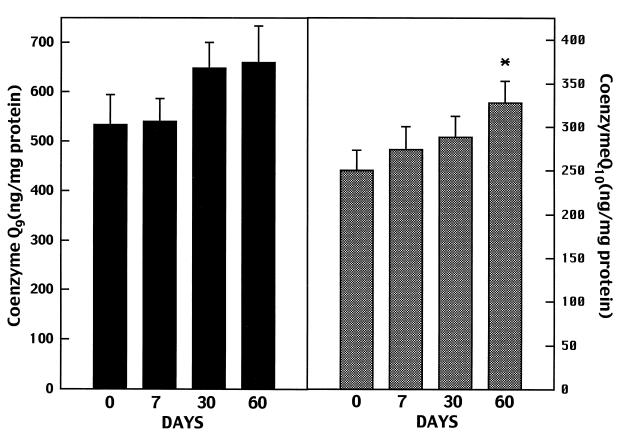
The effects of oral supplementation of coenzyme Q10 on cerebral cortex mitochondria levels of total coenzyme Q9 and total coenzyme Q10 in 12-month-old rats are shown in Fig. 4. Oral supplementation with coenzyme Q10 resulted in progressive increases in brain mitochondrial concentrations of coenzyme Q10 at 7, 30, and 60 days, with increases at 60 days being significant (P < 0.05) as compared with levels in controls. Coenzyme Q9 levels also increased, but the increases were not significant. Vitamin E levels increased from 136.8 ± 24.8 ng/mg of protein at 0 days to 157.9 ± 24.6 ng/mg of protein at 7 days, to 164.7 ± 20.6 ng/mg of protein at 30 days, and to 176.4 ± 15.4 ng/mg of protein at 60 days, but the increases were not significant. In 24-month-old Fisher 344 rats fed with coenzyme Q10 for 1 month, total coenzyme Q10 corrected for mitochondrial citrate synthase significantly increased from 0.005 ± 0.001 to 0.007 ± 0.001 (ng/nmol of citrate synthase/mg of protein per min, P < 0.05).
Figure 4.
Cerebral cortex mitochondrial total coenzyme Q9 and total coenzyme Q10 concentrations in 12-month-old rats treated for 2 months with 200 mg/kg of coenzyme Q10.
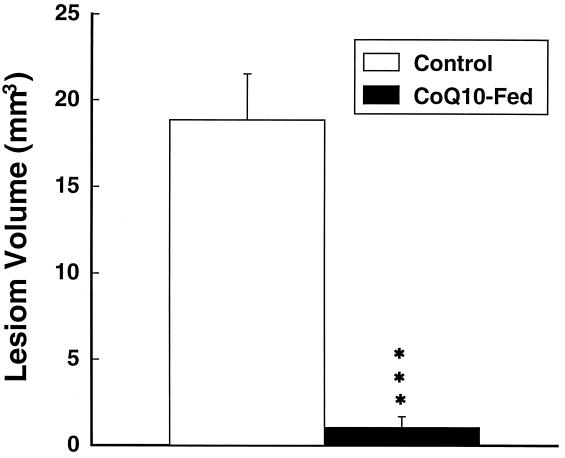
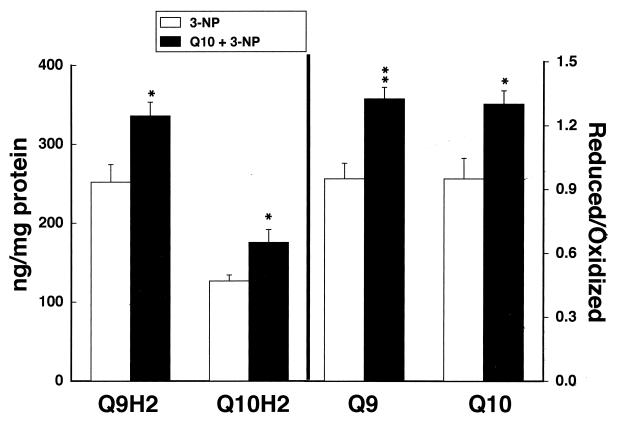
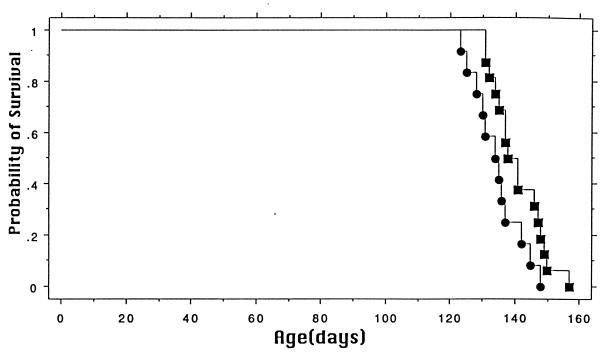
Coenzyme Q10 supplementation for 1 week before administration of 3-NP dramatically reduced the size of the lesions in the treated animals (Fig. 5). Oral supplementation with coenzyme Q10 also significantly attenuated reductions in reduced coenzyme Q9 and reduced coenzyme Q10 after 3-NP administration in the same animals (Fig. 6). We also examined the effects of coenzyme Q10 in a transgenic model of familial ALS, expressing high amounts of human SOD carrying the G93A mutation (16). Heterozygote mice were placed on a diet supplemented with either 200 mg/kg of coenzyme Q10 or on a normal diet starting at 50 days of age. As shown in Fig. 7, oral supplementation with coenzyme Q10 produced a significant increase in survival from a mean of 135–141 days, P < 0.05.
Figure 5.
Effects of coenzyme Q10 supplementation on striatal lesion volumes produced by 3-NP. ∗∗∗, P < 0.001.
Figure 6.
Effects of coenzyme Q10 supplementation versus a normal diet on reduced coenzyme Q9 and reduced coenzyme Q10 levels after administration of 3-NP. ∗, P < 0.05; ∗∗, P < 0.01.
Figure 7.
Effects of coenzyme Q10 supplementation on survival in a transgenic animal model of ALS. The graph shows the cumulative probability for survival. Survival was significantly increased in mice receiving coenzyme Q10. P < 0.05.
DISCUSSION
Coenzyme Q10 is a molecule that acts as both an essential cofactor of the electron transport chain and an endogenous antioxidant (1, 19, 20). Lipid peroxidation leads to a decrease in coenzyme Q10 content and inactivation of respiratory chain enzymes, whereas administration of coenzyme Q10 preserves mitochondrial respiratory function in aged rat skeletal muscle (5, 21). NADPH:quinone oxidoreductase maintains coenzyme Q10 in a reduced state, promoting its antioxidant function (22). Administration of coenzyme Q10 increases the activity of the electron transport chain both in vitro and in vivo and protects against ischemia/reperfusion damage in the heart (23–28) and against damage produced by adriamycin in perfused rat livers (29). Coenzyme Q10 protects against glutamate toxicity in cultured cerebellar neurons (30) and against mumps and sendai virus-induced degeneration of neurons (31).
We previously found that coenzyme Q10 exerts neuroprotective effects in the central nervous system in vivo (13). Oral administration of coenzyme Q10 for 10 days dose-dependently protected against striatal lesions produced by the succinate dehydrogenase inhibitor malonate, and attenuated malonate induced ATP depletions. This finding is consistent with a recent study showing that coenzyme Q10 attenuated decreases in ATP and phosphocreatine produced by ischemia and reperfusion in the heart (28). We also found that coenzyme Q10 administration attenuated striatal lesions produced by aminoxyacetic acid (32), and dopamine depletions produced by 1-methyl-4-phenyl-1,2,5,6 tetrahydropynidine (MPTP) in older mice (33).
An increase in plasma concentrations of coenzyme Q10 after oral supplementation in both humans and animals is well documented. Previous work, however, questioned whether coenzyme Q10 accumulates in tissues (34). In young rats (180–200 g) plasma concentrations of coenzyme Q10 doubled after 4 days of supplementation at 100 mg daily, and there were significant increases in the liver and spleen, but no changes in the heart or kidney (35, 36). Similarly, we and others found no increases in brain concentrations after oral administration of coenzyme Q10 in young (1–2 months old) animals (36, 37). This finding suggests that coenzyme Q10 levels are tightly regulated in young animals and levels in membranes may be saturated. Consistent with this finding myocardial ischemia and reperfusion deplete coenzyme Q9 levels and stimulate coenzyme Q9 synthesis in young, but not old, rats (38).
Coenzyme Q10 levels are known to decrease with aging in both human and rat tissues (17, 18, 39). This decrease may be caused by reduced synthesis or age-dependent increases in lipid peroxidation that can reduce coenzyme Q10 levels (5). Beyer and colleagues (17) found a significant decrease in brain coenzyme Q10 levels as early as 5 months of age (17). It is possible that age-dependent decreases could be restored by dietary supplementation. We therefore examined the effects of dietary supplementation with coenzyme Q10 for 2 months in 12-month-old Sprague–Dawley rats and for 1 month in 24-month-old Fisher 344 rats. After supplementation with coenzyme Q10 there were significant increases in oxidized coenzyme Q9 and coenzyme Q10 as well as their reduced forms in cerebral tissue. Coenzyme Q10 supplementation resulted in 30–40% increases in 12-month-old Sprague–Dawley rats, restoring levels to those seen in the young animals. In 24-month-old Fisher 344 rats we also found a significant increase in coenzyme Q10 levels after feeding for 1 month; however, in those rats the increase was only about 10%, perhaps reflecting strain differences as well as the duration of treatment. We examined brain mitochondrial concentrations of coenzyme Q10 because coenzyme Q10 is particularly concentrated in mitochondrial membranes, where it serves as an important antioxidant (4, 5, 35). Coenzyme Q10 supplementation resulted in significant increases in mitochondrial total coenzyme Q10 concentrations. There was a nonsignificant trend toward an increase in vitamin E concentrations at 30 days, consistent with a vitamin E sparing effect that was observed in other studies (35). These results therefore provide evidence that oral supplementation with coenzyme Q10 can increase brain concentrations in adult animals.
We examined whether coenzyme Q10 could attenuate striatal lesions produced by 3-NP. Systemic administration of 3-NP, an irreversible inhibitor of succinate dehydrogenase, produces selective striatal lesions in both rats and primates that closely resemble those found in Huntington’s disease. In primates the toxin also produces a choreiform movement disorder and frontal-type cognitive deficits (32, 40). The pathogenesis of the lesions involves both impaired energy metabolism and oxidative stress (41). The present results show that coenzyme Q10 is highly effective in attenuating 3-NP neurotoxicity. Furthermore, coenzyme Q10 supplementation maintained significantly higher levels of reduced coenzyme Q9 and coenzyme Q10 after administration of 3-NP as compared with rats on unsupplemented diets. This finding provides further evidence that coenzyme Q10 might be useful in the treatment of Huntington’s disease.
We also examined whether coenzyme Q10 supplementation could exert neuroprotective effects in a transgenic mouse model of familial ALS (Fig. 7). A major advance in understanding ALS was the finding that a subset of families with autosomal-dominant inherited ALS harbor point mutations in the enzyme SOD1 (42). Substantial evidence suggests that these point mutations result in a gain of function of the mutant enzyme that may be caused by oxidative stress (43). Overexpression of the mutant enzyme in transgenic mice leads to motor neuron degeneration whereas overexpression of wild-type human SOD1 does not (16). We found increased levels of 3-nitrotyrosine, a marker of peroxynitrite induced damage in transgenic ALS mice (44, 45). An early pathologic finding in these mice as well as in other transgenic SOD1 mutation mice is mitochondrial swelling and vacuolization (16, 46), suggesting that mitochondrial dysfunction may contribute to pathogenesis. In the present experiments we found that oral administration of coenzyme Q10 significantly increased the life span of transgenic mice with the human G93A SOD1 mutation. Both antioxidant effects and preservation of mitochondrial function may contribute to the observed neuroprotection. Although vitamin E supplementation delays disease onset in G93A mice it has no effect on survival (47). This finding suggests that coenzyme Q10 may be a more effective strategy than vitamin E in the treatment of neurodegenerative diseases.
Studies of coenzyme Q10 levels in Parkinson’s disease platelets show reduced levels that correlate with decreases in mitochondrial complex I activity (48). Coenzyme Q10 administration reduces elevated cortical and basal ganglia lactate concentrations in Huntington’s disease patients as assessed by using magnetic resonance spectroscopy (49). The present results show that oral administration of coenzyme Q10 increases brain mitochondrial concentrations and produces neuroprotective effects in animal models of Huntington’s disease and ALS, strengthening the prospect that coenzyme Q10 may be a useful treatment for neurodegenerative diseases.
Figure 2.
Cerebral cortex concentrations of reduced coenzyme Q9 H2 and coenzyme Q10 H2 in 12-month-old rats treated with 200 mg/kg of coenzyme Q10. ∗∗, P < 0.01.
Acknowledgments
The secretarial assistance of Sharon Melanson is gratefully acknowledged. This work was supported by National Institutes of Health Grants PO1 AG12992, NS16367, NS31579, and NS32365.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ALS, amyotrophic lateral sclerosis; SOD, superoxide dismutase; 3-NP, 3-nitropropionic acid.
References
- 1.Beyer R E. Biochem Cell Biol. 1992;70:390–403. doi: 10.1139/o92-061. [DOI] [PubMed] [Google Scholar]
- 2.Ernster L, Dallner G. Biochim Biophys Acta. 1995;127:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 3.Do T Q, Schultz J R, Clarke C F. Proc Natl Acad Sci USA. 1996;93:7534–7539. doi: 10.1073/pnas.93.15.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noack H, Kube U, Augustin W. Free Radical Res. 1994;20:375–386. doi: 10.3109/10715769409145637. [DOI] [PubMed] [Google Scholar]
- 5.Forsmark-Andree P, Lee C-P, Dallner G, Ernster L. Free Radical Biol Med. 1997;22:391–400. doi: 10.1016/s0891-5849(96)00330-9. [DOI] [PubMed] [Google Scholar]
- 6.Abe K, Fujimura H, Nishikawa Y, Yorifuki S, Mezaki T, Hirono N, Nishitani N, Kameyama M. Acta Neurol Scand. 1991;83:356–359. doi: 10.1111/j.1600-0404.1991.tb03962.x. [DOI] [PubMed] [Google Scholar]
- 7.Bresolin N, Bet L, Binda A, Moggio M, Comi G, Nador F, Ferrante C, Carenzi A, Scarlato G. Neurology. 1988;38:892–899. doi: 10.1212/wnl.38.6.892. [DOI] [PubMed] [Google Scholar]
- 8.Ihara Y, Namba R, Kuroda S, Sato T, Shirabe T. J Neurol Sci. 1989;90:263–271. doi: 10.1016/0022-510x(89)90112-3. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa Y, Takahashi M, Yorifuji S, Nakamura Y, Ueno S, Tarui S, Kozuka T, Nishimura T. Neurology. 1989;39:399–403. doi: 10.1212/wnl.39.3.399. [DOI] [PubMed] [Google Scholar]
- 10.Shoffner J M, Lott M T, Voljavec A S, Soueidan S A, Costigan D A, Wallace D C. Proc Natl Acad Sci USA. 1989;86:7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beal M F. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 12.Beal M F. Ann Neurol. 1992;31:119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- 13.Beal M F, Henshaw R, Jenkins B G, Rosen B R, Schulz J B. Ann Neurol. 1994;36:882–888. doi: 10.1002/ana.410360613. [DOI] [PubMed] [Google Scholar]
- 14.Mutisya E M, Bowling A C, Beal M F. J Neurochem. 1994;63:2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 15.Schulz J B, Henshaw D R, Matthews R T, Beal M F. Exp Neurol. 1995;132:279–283. doi: 10.1016/0014-4886(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 16.Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H-X, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 17.Beyer R E, Burnett B-A, Cartwright K J, Edington D E, Falzon M J, Kreitman K R, Kuhn T W, Ramp B J, Rhee S Y S, Rosenwasser M J, et al. Mech Aging Dev. 1985;32:267–281. doi: 10.1016/0047-6374(85)90085-5. [DOI] [PubMed] [Google Scholar]
- 18.Kalen A, Appelkvist E-L, Daliner G. Lipids. 1989;24:579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 19.De Jong A M P, Albracht S P J. Eur J Biochem. 1994;222:975–982. doi: 10.1111/j.1432-1033.1994.tb18948.x. [DOI] [PubMed] [Google Scholar]
- 20.Frei B, Kim M C, Ames B N. Proc Natl Acad Sci USA. 1990;87:4879–4883. doi: 10.1073/pnas.87.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiyama S, Yamada K, Ozawa T. Biochem Mol Biol Int. 1995;37:1111–1120. [PubMed] [Google Scholar]
- 22.Landi L, Fiorentini D, Galli M C, Segura-Aguilar J, Beyer R E. Free Radical Biol Med. 1997;22:329–335. doi: 10.1016/s0891-5849(96)00294-8. [DOI] [PubMed] [Google Scholar]
- 23.Sanbe A, Tanonaka K, Niwano Y, Takeo S. J Pharmacol Exp Ther. 1994;269:51–56. [PubMed] [Google Scholar]
- 24.Takeo S, Tanonaka K, Tazuma T, Miyake K, Murai R. J Pharmacol Exp Ther. 1987;243:1131–1138. [PubMed] [Google Scholar]
- 25.Schneider H, Lemaster J J, Hackembroek C R. J Biol Chem. 1982;257:10789–10793. [PubMed] [Google Scholar]
- 26.Nakamura T, Sanma H, Himeno M, Kato K. In: Transfer of Exogenous Coenzyme Q to Inner Membrane of Heart Mitochondria in Rats. Yamamura Y, Folkers K, Ito Y, editors. Vol. 2. Amsterdam: Elsevier; 1989. pp. 3–11. [Google Scholar]
- 27.Ohhara H, Kanaide H, Yoshimura R, Okada M, Nakamura M. J Mol Cell Cardiol. 1981;13:65–74. doi: 10.1016/0022-2828(81)90229-7. [DOI] [PubMed] [Google Scholar]
- 28.Crestanello J A, Kamelgard J, Lingle D M, Mortensen S A, Rhode M, Whitman G J R. J Thorac Cardiovasc Surg. 1996;111:443–450. doi: 10.1016/s0022-5223(96)70455-5. [DOI] [PubMed] [Google Scholar]
- 29.Valls V, Castelluccio C, Fato R, Genova M L, Bovina C, Saez G, Marchetti M, Castelli P, Lenaz G. Biochem Mol Biol Int. 1994;33:633–642. [PubMed] [Google Scholar]
- 30.Favit A, Nicoletti F, Scapagnini U, Canonico P L. J Cereb Blood Flow Metab. 1992;12:638–645. doi: 10.1038/jcbfm.1992.88. [DOI] [PubMed] [Google Scholar]
- 31.Edlund C, Holmberg K, Dallner G, Norrby E, Kristensson K. J Neurochem. 1994;63:634–639. doi: 10.1046/j.1471-4159.1994.63020634.x. [DOI] [PubMed] [Google Scholar]
- 32.Brouillet E, Henshaw D R, Schulz J B, Beal M F. Neurosci Lett. 1994;177:58–62. doi: 10.1016/0304-3940(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 33.Beal M F, Matthews R, Tieleman A, Schults C W. Brain Res. 1997;783:109–114. doi: 10.1016/s0006-8993(97)01192-x. [DOI] [PubMed] [Google Scholar]
- 34.Reahal S, Wrigglesworth J. Drug Metab Dispos. 1992;20:423–427. [PubMed] [Google Scholar]
- 35.Zhang Y, Turunen M, Appelkvist E-L. J Nutr. 1996;126:2089–2097. doi: 10.1093/jn/126.9.2089. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Aberg F, Appelkvist E-L, Dallner G, Ernster L. J Nutr. 1995;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- 37.Beal M F, Matthews R T. Mol Aspects Med. 1997;18:s169–s179. doi: 10.1016/s0098-2997(97)00024-1. [DOI] [PubMed] [Google Scholar]
- 38.Muscari C, Biagetti L, Stefanelli C, Giordano E, Guarnieri C, Caldarera C M. J Mol Cell Cardiol. 1995;27:283–289. doi: 10.1016/s0022-2828(08)80027-2. [DOI] [PubMed] [Google Scholar]
- 39.Battino M, Gorini A, Villa R F, Genova M L, Bovina C, Sassi S, Littarru G P, Lenaz G. Mech Aging Dev. 1995;78:173–187. doi: 10.1016/0047-6374(94)01535-t. [DOI] [PubMed] [Google Scholar]
- 40.Palfi S, Ferrante R J, Brouillet E, Beal M F, Dolan R, Guyoi M C, Peschanski M, Hantraye P. J Neurosci. 1996;16:3019–3025. doi: 10.1523/JNEUROSCI.16-09-03019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz J B, Henshaw D R, MacGarvey U, Beal M F. Neurochem Int. 1996;29:167–171. doi: 10.1016/0197-0186(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 42.Rosen D R, Siddique T, Patterson D, Figiewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan J P, Deng H-X, et al. Nature (London) 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 43.Brown J R H. Cell. 1995;80:687–692. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 44.Bruijn L I, Becher M W, Lee M K, Anderson K L, Jenkins N A, Copeland N G, Sisodia S S, Rothstein J D, Borchelt D R, Price D L, Cleveland D W. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 45.Ferrante R J, Shinobu L A, Schulz J B, Matthews R T, Thomas C E, Kowall N W, Gurney M E, Beal M F. Ann Neurol. 1997;42:326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- 46.Wong P C, Pardo C A, Borchelt D R, Lee M K, Copeland N G, Jenkins N A, Sisodia S S, Cleveland D W, Price D L. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 47.Gurney M E, Cutting F B, Zhai P, Doble A, Taylor C P, Andrus P K, Hall E D. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- 48.Schults C W, Haas R H, Passov D, Beal M F. Ann Neurol. 1997;42:261–264. doi: 10.1002/ana.410420221. [DOI] [PubMed] [Google Scholar]
- 49.Koroshetz W J, Jenkins B G, Rosen B R, Beal M F. Ann Neurol. 1997;41:160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]