Summary
Due to fixational eye movements, the image on the retina is always in motion, even when one views a stationary scene. When an object moves within the scene, the corresponding patch of retina experiences a different motion trajectory than the surrounding region. Certain retinal ganglion cells respond selectively to this condition, when the motion in the cell’s receptive field center is different from that in the surround. Here we show that this response is strongest at the very onset of differential motion, followed by gradual adaptation with a time course of several seconds. Different subregions of a ganglion cell’s receptive field can adapt independently. The circuitry responsible for differential motion adaptation lies in the inner retina. Several candidate mechanisms were tested, and the adaptation most likely results from synaptic depression at the synapse from bipolar to ganglion cell. Similar circuit mechanisms may act more generally to emphasize novel features of a visual stimulus.
Introduction
During normal viewing, the task of detecting moving objects is complicated by the presence of eye movements that continually scan the image across the retina, even during fixation. In the presence of these eye movements (Skavenski et al., 1979; Kowler, 1990) external object motion appears on the retina as differential motion between the patch corresponding to the object and the rest of the retina seeing the background. Thankfully, the visual system has evolved to reliably detect such differential motion, while rejecting the global retinal image motion that is due to eye movements. The process starts already in the retina where certain ganglion cells, termed object motion sensitive (OMS) cells, have the required properties, responding selectively to differential motion between the center of the receptive field and the surround (Ölveczky et al., 2003).
The very onset of object motion is arguably the most relevant feature, as both prey and predator often reveal themselves by initiating movements. The visual system serves us well also in this regard. Our attention is reliably directed towards locations in the scene where motion is initiated (Abrams and Christ, 2003), even on a background of ongoing motion elsewhere and the image slip created by our eye movements. Here we examine how the retina might contribute to this visual performance, by recording the responses of retinal ganglion cells at the very onset of differential image motion on the retina. We find that the response is very strong at the initiation of movement, but undergoes rapid adaptation as differential motion is maintained. Through intracellular recordings from interneurons and by using a set of novel stimuli, we identify a probable cellular mechanism for this adaptation, and gain further insight into the spatial scale of adaptation and the retinal microcircuitry underlying the OMS response.
Results
OMS cells adapt to differential motion
We recorded the spike trains of ganglion cells in the isolated salamander retina. The stimulus display was divided into an Object region covering the ganglion cell’s receptive field center and part of its surround, and a peripheral large Background region covering the rest of the retina (Figure 1A–B). Both object and background were given a visual texture by a simple stripe grating. The background grating jittered laterally with a random walk trajectory, similar to that of fixational eye movements (Manteuffel et al., 1977; Engbert and Kliegl, 2004) (see Methods). The object grating also jittered in a random walk with the same statistics, either coherently with the background (Global Motion) – simulating a stationary background scanned by eye movements – or with a different random trajectory (Differential Motion) – simulating an object moving on a stationary background scanned by eye movements. The trajectories in the object and background regions alternated periodically, every 40 seconds, between Global Motion and Differential Motion. What may seem like a subtle stimulus transition (Figure 1C) simulates a behaviorally important visual event: the initiation of movement within a stationary scene in the presence of fixational eye movements.
Figure 1. Object motion sensitive (OMS) ganglion cells adapt their response to differential motion.
(A) Receptive field profile of an OMS (salamander Fast OFF) ganglion cell. (B) A stripe grating representing an object was projected in and around the cell’s receptive field center, while the remainder of the retina was presented with a background grating. (C) Time course of the gratings plotted along a vertical transect of the display (vertical line in panel B), illustrating the stimuli for Global Motion, Differential Motion, and Local Motion. For clarity, the number of grating bars has been reduced and only 5 seconds are shown for each stimulus condition. The transitions are marked by arrows. (D) Average firing rate of the OMS cell in (A) to 50 successive trials of a stimulus alternating between Global Motion and Differential Motion every 40 s. (E) Firing rate of another OMS cell under alternating Global and Local Motion.
Object motion sensitive ganglion cells respond to such jittering stimuli with sparse bursts of high-frequency firing that are precisely timed to the trajectory (Ölveczky et al., 2003). To gauge a cell’s sensitivity to the stimulus, we measured the average firing rate over many trials with different motion trajectories. The switch from Global to Differential Motion caused, on average, an ~80-fold increase in firing rate (41 OMS cells, 5 retinas, range 7–435, see Figure 1D for an example). During continued exposure to Differential Motion, the OMS cells then reduced their firing rate by, on average, 58% (range 27–78%). By analogy to previous studies on motion adaptation (Clifford and Ibbotson, 2002; Hoffmann et al., 1999; Van Wezel and Britten, 2002), we will refer to this phenomenon as “differential motion adaptation”. The time course of adaptation was well approximated by an exponential decay, with an average time constant of 7.2 s (range 2.6–17.0 s). This time course is similar to what has been measured for contrast adaptation in the retina (Smirnakis et al., 1997), and for motion adaptation in humans (Hoffmann et al., 1999).
The recovery from differential motion adaptation occurred more slowly (Figure 2), with an average time constant of 52 s (range 25–89 s, 6 cells). A recovery that takes substantially longer than the adaptation itself has been seen for motion adaptation in humans (Hoffmann et al., 1999), as well as other types of sensory adaptation (Best and Wilson, 2004; Chung et al., 2002). A similar asymmetry is found for retinal adaptations to other stimulus variables such as the mean intensity (Enroth-Cugell and Shapley, 1973) and contrast (Smirnakis et al., 1997; DeWeese and Zador, 1998).
Figure 2. Recovery from differential motion adaptation.
(A) Firing rate of an OMS cell in response to a stimulus alternating between 40 s of Differential Motion (D) and a varying interval of Global Motion (G). (B) Firing rate at the onset of Differential Motion relative to the final value, plotted as a function of the preceding duration of Global Motion.
Circuit mechanisms underlying differential motion adaptation
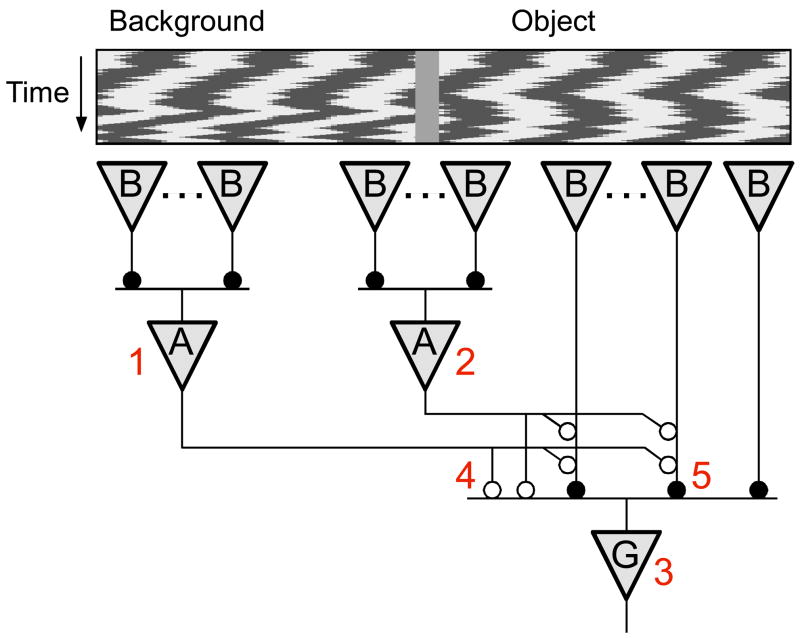
Previous studies (Ölveczky et al., 2003) suggested that the selectivity for differential motion over global motion is accomplished by a specialized circuit (Figure 3). Consider an OMS ganglion cell whose receptive field center lies in the object region. Motion of the object drives an array of bipolar cells, whose rectified output excites the OMS cell. Motion of the background drives bipolar cells in the periphery, which excite a network of polyaxonal amacrine cells. Inhibition from these wide-field amacrine cells combines with excitation from local bipolar cells, either presynaptically on the bipolar cell terminals or directly at the OMS ganglion cells. The dynamics and non-linearities in the circuit operate such that under global motion the inhibition cancels the excitation and the OMS cell remains silent (Ölveczky et al., 2003). Where within this circuitry does the adaptation to differential motion occur, and what are the neural mechanisms involved?
Figure 3. Neural circuitry underlying object motion sensitivity.
The OMS ganglion cell (G) receives excitatory input through rectifying synapses from multiple bipolar cells (B). OMS cells are inhibited both directly and indirectly by amacrine cells (A). Numbers represent sites potentially involved in differential motion adaptation. 1 – The inhibitory surround region. 2 – A polyaxonal amacrine cell spanning object and background regions. 3 – The OMS cell. 4 – The inhibitory synapse from amacrine cells to the OMS ganglion cell. 5 – The excitatory synapse from bipolar cells to the OMS ganglion cell.
Given the observed similarities in the time course of differential motion adaptation and contrast adaptation, it is worth reviewing first what has been learned about the mechanisms of contrast adaptation. Following an increase in the strength of a visual stimulus, for example the contrast of a flickering spot, the sensitivity of a retinal ganglion cell gradually declines (Smirnakis et al., 1997). The outer retina –photoreceptors and horizontal cells – is not involved in this gain change (Rieke, 2001; Baccus and Meister, 2002). A portion of the change already occurs in the bipolar cells that provide excitatory input to the ganglion cell (Rieke, 2001). Another part of the effect is a gain change intrinsic to the ganglion cell itself (Kim and Rieke, 2003; Manookin and Demb, 2006). However, most of the gain change occurs somewhere prior to transmitter release from the bipolar cells, and is thought to be triggered by a strong increase in bipolar cell stimulation (Brown and Masland, 2001; Kim and Rieke, 2001; Manookin and Demb, 2006). Inhibition from amacrine cells seems to play no role in this (Brown and Masland, 2001; Manookin and Demb, 2006).
Differential motion adaptation differs from this phenomenology in two crucial aspects. First, the onset of differential motion produces no overt change in stimulation of the receptive field center. In fact, all local statistics of the stimulus (mean, contrast, power spectrum) are identical everywhere on the retina. The only change is in the correlation of image motion between center and periphery. As a consequence, the excitation of bipolar cells – thought to be essential for contrast adaptation – remains unchanged between differential and global motion (see also Figure 8). Second, the inhibition from amacrine cells – thought to be irrelevant for contrast adaptation – is essential to even obtain the OMS response (Ölveczky et al., 2003). Thus it is difficult to draw clear predictions from the prior work on contrast adaptation, and we are forced to contemplate various possible sites of adaptation within retinal circuitry.
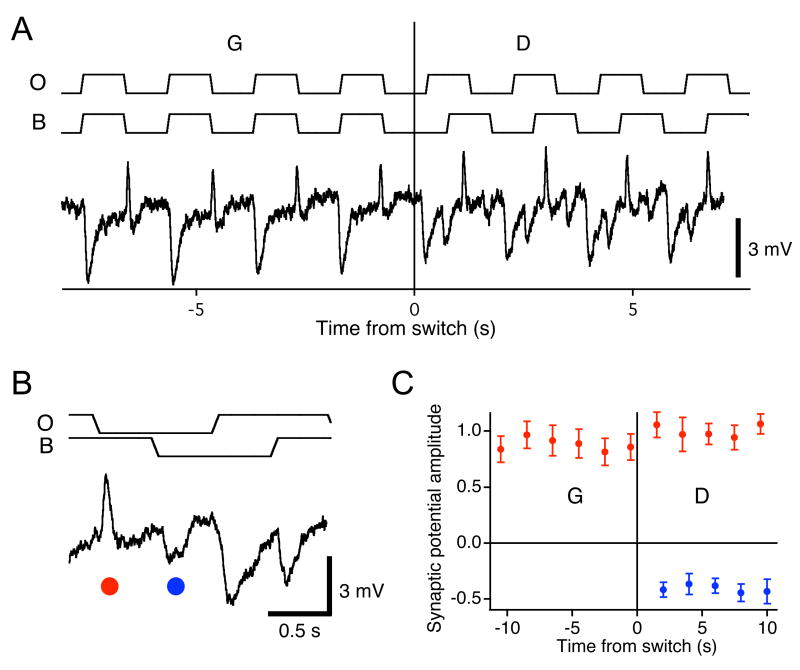
Figure 8. No adaptation in the bipolar cell response.
(A) Membrane potential of an OFF bipolar cell under the periodic shift stimulus of Figure 6A. Average of 3 traces. The receptive field was centered on the object region. Stimulus traces indicate movement of the object (O) and background (B). (B) Enlargement of the trace illustrating excitatory and inhibitory potentials triggered by the grating shifts. (C) The amplitude of the excitatory (red) and inhibitory (blue) potentials marked in panel B as a function of time relative to the switch to Differential Motion. Recordings were obtained from 7 bipolar cells and normalized by the average EPSP during differential motion.
Nevertheless, the nature of the OMS computation allows a restriction of the search. Note that the mere detection of differential motion requires comparing the trajectories in the object and background regions. Consequently, adaptation to differential motion can occur only after the signals from these two regions are combined. Horizontal cells could, in principle, transmit visual signals over long distances to the center, but they hardly respond to the jittering gratings used in these experiments (data not shown). Furthermore, differential motion selectivity in OMS cells requires glycinergic inhibition (Ölveczky et al., 2003). Both observations speak for lateral signal flow via amacrine cells rather than horizontal cells. Thus, the most likely site of adaptation is the inner retina, where spiking glycinergic amacrine cells with long-range connections allow for comparisons between signals from distant regions of the retina (Cook et al., 1998; Ölveczky et al., 2003). Considering the circuitry in Figure 3, the possible sites of adaptation are:
the inhibitory surround;
the polyaxonal amacrine cells in the object region;
the OMS ganglion cells;
the synapse between inhibitory amacrine cells and the OMS ganglion cell;
the synapse between excitatory bipolar cells and the OMS ganglion cell. We designed experiments to probe each of these different possibilities.
1) Does the inhibitory surround adapt to differential motion?
We first examined the role of the inhibitory surround in differential motion adaptation, by using a stimulus that does not activate the surround. Beginning with Global Motion, we switched to Local Motion by halting the motion of the background grating (Figure 1E). This produced a sudden increase in firing of OMS cells, very similar to the transition to Differential Motion. Subsequently, the firing rate declined gradually (Figure 1E) to ~55% of the initial value (range 45 – 80%; 6 cells), with a time constant of ~5.1 s (range 3.8 – 5.9 s). This adaptation closely resembled the one observed under Differential Motion (Figure 1D). Thus while the increase in firing after the switch to Differential Motion is due to a relief of coincident inhibition from the surround region (Ölveczky et al., 2003), the subsequent adaptation occurs equally whether the surround is stimulated or not. This observation greatly simplifies the search for the neural mechanisms underlying differential motion adaptation, as we need consider only neurons processing the stimulus in the center region of the OMS ganglion cell.
2) Do polyaxonal amacrine cells adapt to differential motion?
The suppression of OMS ganglion cells during global motion is likely delivered by polyaxonal amacrine cells (Ölveczky et al., 2003). Those amacrine cells that receive input from both the object and background regions are expected to respond differently to global and differential motion. If this leads to use-dependent changes within the amacrine cell itself, those could play a role in differential motion adaptation.
To test this idea we recorded the intracellular membrane voltage of polyaxonal amacrine cells with somas in the object region under the Differential Motion onset stimulus (Figure 4A). The membrane potential fluctuations of these neurons were significantly larger during Global Motion than Differential Motion. This confirms that the amacrine cell receives input from both object and background. During Global Motion, input from the background is synergistic with input from the object region, making it a highly effective stimulus. However, following the onset of Differential Motion, there was no gradual change in the amacrine cell’s response (the fractional change of the membrane potential standard deviation was 0.003 ± 0.014; n=6). This shows that the amacrine cell does not itself adapt after switching to Differential Motion, and effectively rules out the intrinsic properties of polyaxonal amacrine cells as contributors to differential motion adaptation.
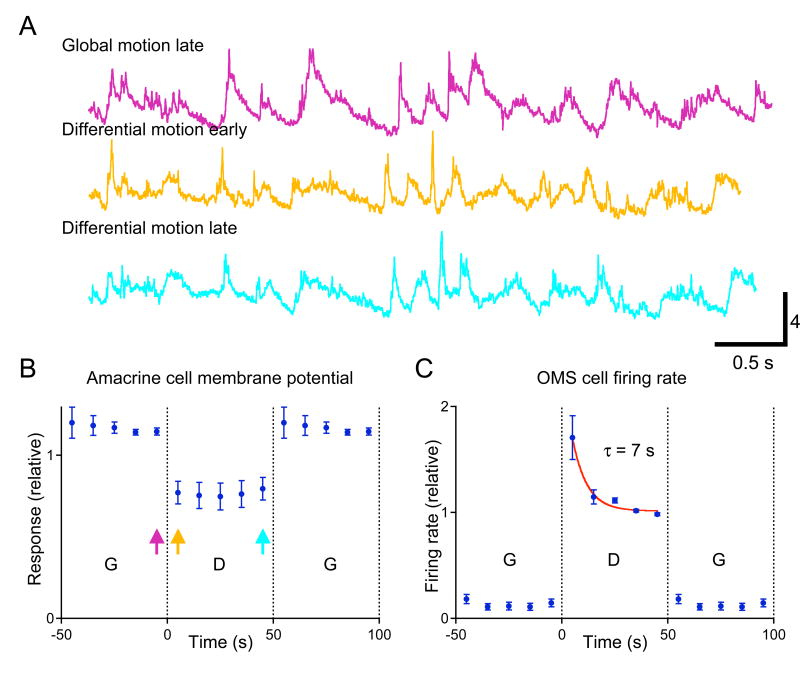
Figure 4. Polyaxonal amacrine cells do not adapt to differential motion.
(A) Membrane potential response of a polyaxonal amacrine cell to the same object trajectory during different phases of the stimulus; see corresponding arrows in (B). The object region experienced a 10-s random motion trajectory, repeated identically every 10 s. The background region alternated between Global and Differential Motion every 50 s (see Methods). (B) Standard deviation in the membrane potential of a polyaxonal amacrine cell under switching between Global and Differential Motion. Response averaged over 4 trials of the same stimulus normalized by the standard deviation over the entire response. (C) The average firing rate of 6 OMS cells in this retina under the same stimulus.
3) Is adaptation intrinsic to OMS cells?
Given that OMS cells increase their firing dramatically after switching to Differential Motion (Figure 1), the adaptation that follows could, in principle, be due to dynamic, spike-dependent changes in the OMS cell’s membrane properties. For example, if an additional membrane conductance develops over the adaptation period, then the same synaptic input currents will lead to smaller membrane voltage excursions, and thus less spiking (Sanchez-Vives et al., 2000). During the Global Motion period, one would expect this conductance to decrease again, leading to a gradual increase in the voltage excursions. We tested this by recording the intracellular membrane potential of OMS ganglion cells (Figure 5), measuring the standard deviation of the stimulus-evoked subthreshold activity.
Figure 5. OMS ganglion cell response does not change during global motion.
(A) Membrane potential response of an OMS ganglion cell to the same object trajectory during different phases of the stimulus; see corresponding arrows in (B). The object region experienced a 5-s random motion trajectory, repeated identically every 5 s. The background region alternated between Global and Differential Motion every 50 s. (B) Response of an OMS ganglion cell to the differential motion onset stimulus: firing rate (left axis) and standard deviation of the subthreshold membrane potential (right axis). Note the adaptation in response to differential motion, but the lack of recovery during global motion. This could be explained if the OMS cell receives two types of bipolar cell input: The dotted line indicates a hypothetical component that is identical under Global and Differential Motion; the dashed line denotes a component that is active only during Differential Motion and declines in strength (see text for detail). Baseline noise of 0.81 mV has been subtracted from the membrane potential fluctuations to yield the stimulus-driven response.
Upon a switch to Differential Motion, membrane voltage fluctuations increased immediately, and then declined gradually by a factor of 0.24 ± 0.03 (5 cells) in the course of adaptation (Figure 5B). With the subsequent switch to Global Motion, voltage fluctuations dropped immediately. However, there was no gradual recovery of subthreshold activity during this period; it remained constant to within a fraction of 0.01 ± 0.02. Likewise, the spiking output of OMS cells showed no significant recovery during the Global Motion phase: from 0.23 ± 0.05 Hz early on (0 – 4 s, 41 cells) to 0.21 ± 0.05 Hz later (36 – 40 s).
How can one reconcile the observed adaptation during Differential Motion with the lack of any recovery during Global Motion? An activity-dependent change in a membrane conductance can account for this only if that conductance is used exclusively during Differential Motion. For example, if the adapting membrane current is voltage-dependent (Kim and Rieke, 2003) with a high threshold, then only the large fluctuations during Differential Motion might be affected by adaptation.
However, there is an alternative explanation in which the ganglion cell’s membrane properties remain constant, but the synaptic inputs from bipolar cells adapt (Figure 5B). In this scenario, one set of bipolar cell terminals is very active during Differential Motion and gradually loses strength. During Global Motion these terminals are silent and make no contribution to the membrane potential, but gradually recover their synaptic strength. A second set of bipolar cell inputs retains constant strength throughout Global and Differential Motion. The sum of the two inputs yields a constant response during Global Motion, but an adapting response during Differential Motion. This idea will receive additional support in the following sections.
4 & 5) Does the pathway from amacrine cells to OMS ganglion cells adapt?
It appears that adaptation to differential motion is not explained by intrinsic cellular mechanisms in either the OMS cell or the polyaxonal amacrine cell. Given the working hypothesis for the OMS circuit (Figure 3), alternative sites of adaptation are the synapses that provide excitation or inhibition to the OMS cells. We first probed for dynamic changes in the interactions between inhibitory amacrine cells and the OMS ganglion cell. If synapses on the path from an amacrine to a ganglion cell were to change in strength, then the resulting adaptation occurs before the contributions from all the peripheral amacrine cells are pooled at the level of the OMS cell. We designed a stimulus specifically to test this prediction.
Rather than jitter the object and background gratings randomly according to fixational eye-movements, we shifted them periodically, as this afforded complete control over the correlations between the synaptic inputs from different parts of the receptive field (Figure 6A). When the gratings in the object and background regions were shifted in synchrony (Global Motion), OMS cells typically remained silent. When the gratings shifted asynchronously (Differential Motion), movement of the object grating caused the cell to fire a rapid burst of spikes (Figure 6A). The amplitude of those bursts declined gradually (Figure 6B), replicating the differential motion adaptation seen with continuously jittering gratings (Figure 1).
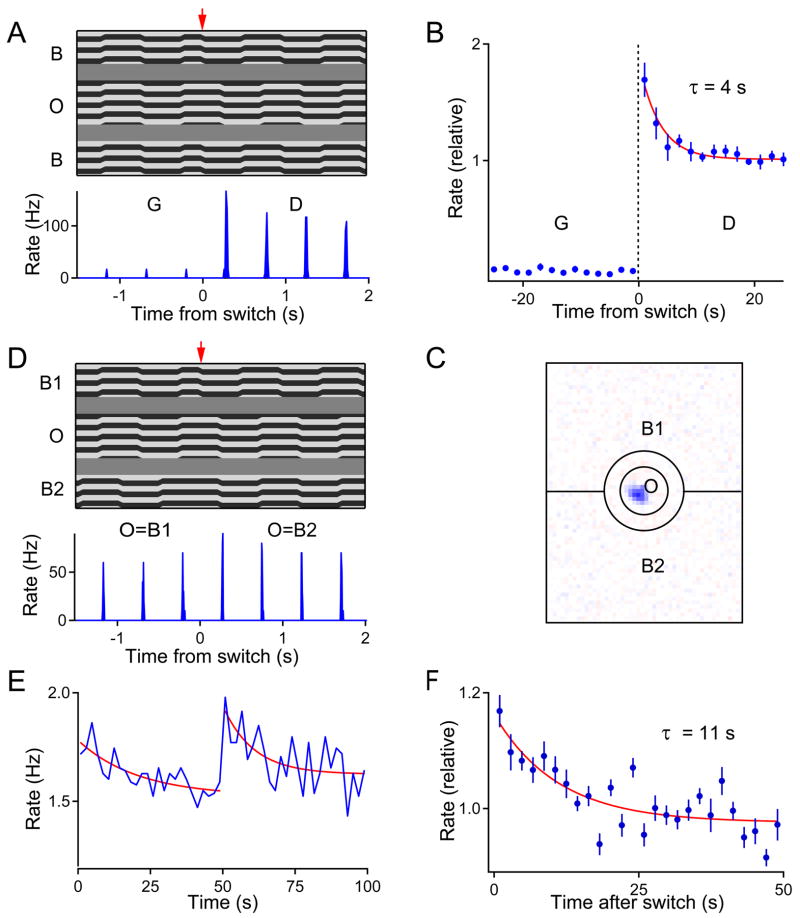
Figure 6. Differential motion adaptation happens before spatial summation of the surround.
(A–B) A simplified differential motion onset stimulus elicits similar response in OMS ganglion cells as the random jitter stimulus (Figure 1). (A) Top: Motion trajectories for the ‘grating shift’ stimulus, presented as in Figure 1C. An object grating (O) and a background grating (B) shifted back and forth 13 μm every 0.5 s. The gratings shifted in synchrony for Global Motion (G) and in alternation for Differential Motion (D). The arrow marks the switch between the two conditions. Bottom: Firing rate of an OMS cell in response to the above stimulus. Average over 30 trials. (B) Responses to this stimulus averaged over 12 OMS cells. Each data point reflects the firing during 2 successive grating shifts. (C) Outline of the ‘split surround’ stimulus, drawn on the receptive field of an OMS ganglion cell. Again a circular object region (O) covered the receptive field center. The background was divided into two halves, B1 and B2. All 3 regions were painted with striped gratings (not shown). (D) Top: Motion trajectories for the ‘split surround’ stimulus. One of the background regions stepped in synchrony with the object, the other in alternation. Every 50 s the two regions swapped roles. This transition is marked by the arrow. The step size was 27 μm. Bottom: Firing rate of an OMS cell in response to this stimulus. Average over 20 trials. (E) Response of an OMS cell to the ‘split surround’ stimulus averaged across 20 trials. Each data point reflects the firing during 2 successive grating shifts. (F) Responses averaged over 4 OMS cells and both phases of the stimulus.
To test whether this adaptation happens before the summation of inhibitory inputs, the background region was split into two equal halves: In one half, the grating shifted in phase with the object grating; in the other half, the grating shifted out of phase with the object (Figure 6C–D). Every 50 seconds, the two halves of the background swapped roles. Thus, at any given time, the OMS cell experienced Global Motion with respect to half of the background but Differential Motion with respect to the other half. For many OMS cells, Global Motion of only half the background was not sufficient to completely suppress firing, and a burst was observed on every shift of the object grating (Figure 6D). When the two halves of the background switched roles, this response increased immediately – by 20% on average – then adapted gradually back to a steady level (Figure 6E–F). This shows that there is significant adaptation in the OMS response prior to spatial summation of the surround. Therefore, adaptive changes do indeed occur along the synaptic pathway from the peripheral amacrine cells to the OMS cell. Two plausible sites for this modulation need to be considered.
One possibility is a direct synapse between the polyaxonal amacrine cell and the OMS ganglion cell (site 4 in Figure 3). When the two halves of the background swap phases (Figure 6D), the activity of the individual peripheral amacrine cell is expected to stay the same, but its correlation with the OMS cell in the object region changes. If the synaptic strength of this synapse is modulated by the correlations between presynaptic and postsynaptic signals in an anti-Hebbian fashion (Aizenman et al., 2000) then one could explain the gradual decline of the OMS firing rate (Figure 6F).
However, amacrine cells can also interact indirectly with the OMS cells, by inhibiting bipolar cell terminals in the receptive field center (Cook et al., 1998) (site 5 in Figure 3). This offers another potential mechanism for differential motion adaptation: presynaptic depression. In this picture, during Global Motion the peripheral amacrine cells inhibit a bipolar cell terminal whenever the bipolar cell depolarizes. This will reduce or eliminate synaptic transmitter release, and thus the excitatory drive to the ganglion cell. With a switch to Differential Motion, the bipolar cell depolarizes in the same way, but there is no coincident presynaptic inhibition. Because the synapses are primed and release-ready, the bipolar terminal immediately provides a large drive to the OMS cell. If the synapse depresses under repetitive use (von Gersdorff and Matthews, 1997; Burrone and Lagnado, 2000), its output will gradually decline, leading to the observed adaptation in the OMS cell response. To explain adaptation to the ‘split-surround’ stimulus (Figure 6), one needs to assume further that individual bipolar cell terminals receive asymmetric inhibition dominated by one or the other half of the background, perhaps as a result of random connections to the processes of wide-field amacrine cells. This hypothesis led to the following experiments.
5) Is depression at the bipolar cell terminal involved in adaptation?
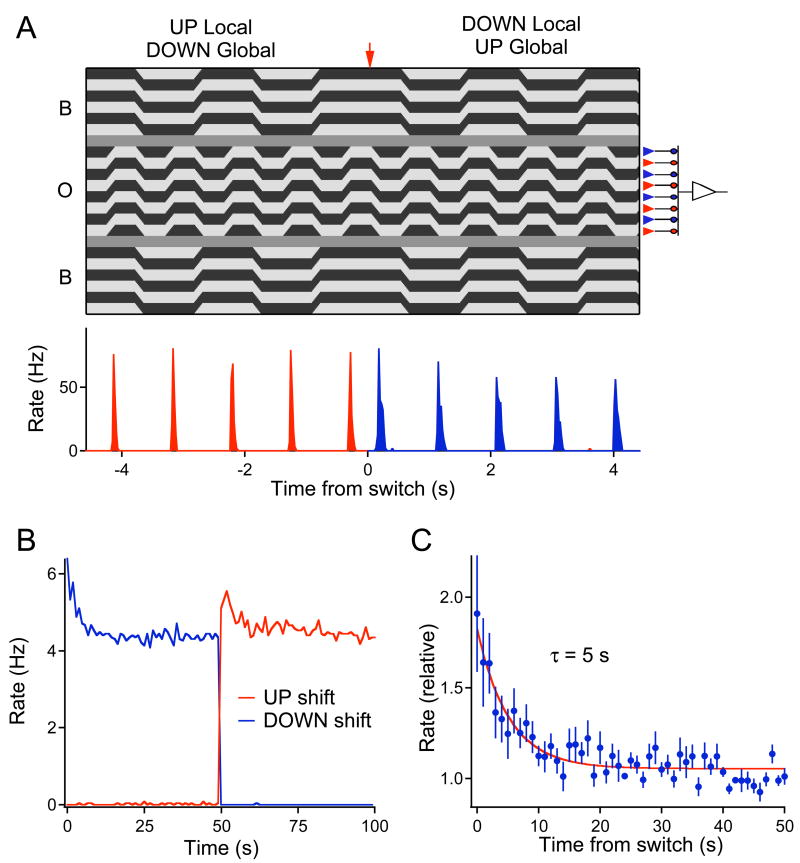
If the site of adaptation is indeed the bipolar-to-ganglion cell synapse, then adaptation to differential motion must occur before the input from the bipolar cell population is summed at the level of the ganglion cell. To examine this directly, we exploited a stimulus that can drive bipolar and ganglion cells independently. We used a fine object grating with bars of 67 μm width, about the size of bipolar cell receptive fields (Hare and Owen, 1996; Ölveczky et al., 2003). This grating was shifted back and forth by one bar width, ensuring that a different subset of OFF-type bipolar cells was excited on consecutive shifts of the grating (Figure 7A). The background grating shifted at only half the frequency, and was in phase either with the upward or the downward shifts of the object grating. Every 50 s, this phase was swapped. Under this stimulus, an individual bipolar cell experiences inhibition from amacrine cells in synchrony with excitation from photoreceptors for 50 s. In the following 50 s, the inhibition is out-of-phase with the excitation. The OMS ganglion cell, which sums over many bipolar cells in the object region, experiences the same amount of differential motion at all times. Therefore, if adaptation happens after the OMS cell has summed its inputs, then the switch in the phase of the background grating should not yield any changes in the firing rate. On the other hand, if adaptation is due to depression at individual bipolar cell terminals, then the switch should produce a transient increase in OMS cell firing, because a previously silent set of bipolar terminals suddenly gets activated.
Figure 7. Differential motion adaptation happens before spatial summation of the center.
(A) Top: Stimulus designed to probe adaptation at the bipolar cell terminals. The object grating shifted back and forth by one bar width (67 μm) at 2 Hz. The background grating shifted at 1 Hz, in synchrony with the downward shifts of the object grating; 50 s later, the background switched phase to synchronize with upward shifts of the object. The transition is marked by the arrow. A simplified circuit diagram (right) illustrates how the up- and down-shifts of the object grating drive two distinct populations of bipolar cells, thereby separating their inputs to the ganglion cell in time. Bottom: Firing rate of an OMS cell in response to the above stimulus. Average over 30 trials. (B) Response of an OMS ganglion cell to the stimulus in (A) averaged over 30 trials. Each data point reflects the average firing rate during one shift of the object grating. In the interval 0–50 s the background shifts coincided with upward object shifts, in 50–100 s with downward object shifts. (C) Responses averaged over 12 OMS cells and both phases of the stimulus.
The results were consistent with the latter hypothesis. As expected from preceding experiments, the OMS cells fired vigorously on the Differential Motion shifts of the object grating, but remained essentially silent during the Global Motion shifts (Figure 7A). More importantly, when the phase of the background grating switched, the Differential Motion response increased suddenly, then declined back to the steady-state level (Figure 7B). On average, the increase amounted to a factor of 2 and the time constant of subsequent adaptation was ~5 s (12 OMS cells, Figure 7C). Note that the magnitude and the time course of this effect match the overall motion adaptation phenomenon (Figures 1D–E, 4C, 6B).
This result shows that differential motion adaptation occurs in large part before spatial summation of the excitatory inputs to the ganglion cell, and is consistent with a form of depression at the bipolar-to-ganglion cell synapse (site 5 in Figure 3). By contrast, plasticity at the amacrine-to-ganglion cell synapse (site 4 in Figure 3) cannot explain the adaptation seen in Figure 7. Polyaxonal amacrine cells have large receptive fields that pool over many bipolar cells, and they respond identically to gratings of opposite contrast (Ölveczky et al., 2003). Consequently the inhibitory synapse between the amacrine and ganglion cell will not experience any change when the background grating switches phase. By the same arguments, adaptation of the intrinsic properties of amacrine cells (site 2 in Figure 3) or of ganglion cells (site 3) cannot account for the observations in Figure 7. Neither of those two sites would experience a change in activity after the stimulus switch if the bipolar synapses retain constant strength.
To be confident that adaptation derives from plasticity in the bipolar cell’s transmitter release mechanism, one would like to confirm that the inputs to the synaptic terminal do not themselves undergo any adaptation. Excitatory input to the bipolar cell comes exclusively from the object region, and therefore does not change at the transition to differential motion. However, the bipolar cell terminal does receive input from the background region, and this could somehow alter the cell’s sensitivity to its excitatory input. Moreover, local polyaxonal amacrine cells receive inputs from both object and background regions, and thus change their response at the transition to Differential Motion (Figure 4); in principle this could lead to a gradual change in their synaptic transmission to the bipolar cell terminal.
To test these possibilities, we recorded directly from OFF bipolar cells under the same periodic shift stimuli (Figure 8). This allowed a separate measurement of central excitation and peripheral inhibition during retinal adaptation. The bipolar cell soma depolarized when a dark bar shifted into its receptive field, and hyperpolarized when the bright bar moved in half a period later. Under Differential Motion, an inhibitory post-synaptic potential was triggered by each shift of the background grating, likely reflecting the inhibitory input from amacrine cells on the synaptic terminal. Neither the excitatory nor the inhibitory potentials showed any time-dependent change after the switch to Differential Motion (Figure 8c). Consequently, it appears that the inputs to the synaptic terminal are indeed constant over time. Though this was a consistent finding in all the bipolar cells we inspected, there remains the possibility that we missed a special type of bipolar cell that drives OMS cells and behaves differently.
Closer inspection of the bipolar cell response (Figure 8a) shows that the depolarization from the object shift is essentially unchanged whether or not there is simultaneous inhibitory input from the background shift. This suggests that the site of recording is electrotonically close to the dendrites, and the strong excitatory conductance during the object shift essentially clamps the somatic potential (Koch et al., 1983; Vu and Krasne, 1992). It is expected that near the axon terminal the reverse occurs, such that the strong inhibitory conductance cancels electrotonically distant excitation, and effectively blocks transmitter release. These interpretations would benefit greatly from direct observation of electrical activity at the terminals.
Ethological role of differential motion adaptation
In what way might adaptation to differential motion be beneficial for the organism, and thus adaptive in the strict sense? If part of the retinal image contains continuous real-world motion for several seconds, such as swaying leaves or rippling water, this region’s differential motion signals will gradually attenuate. Meanwhile other parts of the retina retain high sensitivity to signal the onset of new motion, which is potentially of greater survival interest. On other occasions, the entire retina may become adapted, for example as a result of self-motion through a patterned environment. What consequences, other than a reduction in sensitivity, will such adaptation have on the representation of moving objects?
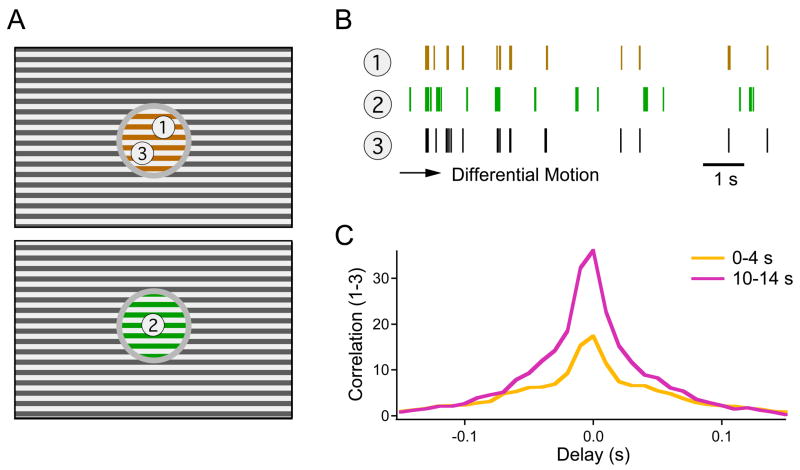
As shown previously, the spike times of an OMS ganglion cell are determined very reliably by the motion trajectory in its receptive field center (Ölveczky et al., 2003). A rigidly moving object produces the same image motion, and thus almost the same spike train in all the OMS cells it covers. Thus, synchronous firing in the OMS cell population may act as a tag that binds together image regions belonging to the same object (Ölveczky et al., 2003). But the detection of this stimulus-driven synchrony is more ambiguous if firing rates in the population are high, since that increases the probability of spurious spike coincidences. On this background, adaptation to differential motion may serve to reduce such ambiguity: As the spike trains become sparser, spurious coincidences between cells seeing differently moving objects would become rarer, and the synchronous events between cells seeing the same object more significant (Figure 9).
Figure 9. Adaptation increases the correlation between OMS cells that view the same object.
(A) Scenario with two moving objects following different trajectories (represented by different colors) and an independently jittered background. (B) Spike trains recorded from three OMS cells, two of them (1 and 3) seeing the same motion trajectory. In the course of adaptation to Differential Motion, the firing events gradually become sparser. (C) Cross-correlation function between the spike trains of two cells viewing the same object (see Methods). This represents the rate of spike coincidences at a given delay, divided by the spurious rate of such coincidences if the same cells were driven by independent objects.
To test this notion, we monitored synchronous firing among OMS neurons at various times after the switch to Differential Motion. As predicted, the same-object correlations indeed became more significant in the course of adaptation (Figure 9). The ratio of spike synchrony from the same object vs different objects (see Methods) increased by 34 ± 16% over the first 10 s of adaptation (mean ± SD; 28 cell pairs). Thus, while a burst of OMS cell activity serves to rapidly indicate where an object starts to move, subsequent adaptation may make the process of discerning object identity based on synchronous firing more reliable and robust. Neural coding strategies where the details of a particular stimulus are revealed robustly only after a dynamic sparsening of the response have also been proposed for olfaction (Laurent, 2002) and face recognition (Sugase et al., 1999).
Discussion
This study was aimed at understanding the responses of retinal neurons when an object in the scene begins to move on the background of the ever-present image motion caused by fixational eye-movements. We focused on the OMS ganglion cells described previously (Ölveczky et al., 2003), and found that:
The OMS cells respond to the onset of differential motion with a dramatic increase in firing rate, which subsequently adapts with a time constant of ~7 seconds, reducing the steady-state firing rate to less than half its initial value.
Subregions of the ganglion cell receptive field, corresponding in size to individual bipolar cells, can adapt independently to differential motion. However, the bipolar cell membrane potential itself shows no sign of adaptation.
The primary cellular mechanism of this adaptation is likely synaptic depression at the OFF-bipolar cell terminal, whose activity is controlled by presynaptic inhibition from polyaxonal amacrine cells. Other potential sites of cellular or synaptic modulation contribute little to differential motion adaptation.
One functional consequence of this adaptation is an increase in the precision of a synchrony code, by which a population of OMS ganglion cells can tag different regions of the same moving object.
Absolute motion and differential motion
Many neurons in the retina and elsewhere in the visual system respond preferentially to moving stimuli (Clifford and Ibbotson, 2002; Taylor and Vaney, 2003). If image motion persists for some time, these responses decline in strength (Barlow and Hill, 1963; Giaschi et al., 1993; Van Wezel and Britten, 2002), and such adaptation has been invoked as a neural substrate for motion aftereffects like the waterfall illusion (Barlow and Hill, 1963). In these studies of absolute motion, the stimulus onset always entails a strong change in the visual input to the neuron’s receptive field center, and thus the ensuing adaptation may well result from local mechanisms within the center’s circuitry (Brown and Masland, 2001). By contrast, the study of differential motion involves more subtle stimuli. In the experiments described here, any local region of the retina always experiences the same amount of motion. The observed response in OMS ganglion cells depends entirely on a comparison between the motion trajectory in the cell’s center and surround. Correspondingly, the circuit elements responsible for adaptive effects must lie after convergence of signals from the receptive field center and surround. Based on prior work it appears that the relevant surround signals are carried by wide-field amacrine cells in the inner retina, not by horizontal cells in the outer retina (Ölveczky et al., 2003).
Interaction of center and surround
We searched for differential motion adaptation at several candidate sites in the retina’s circuit that have access to input from both center and surround (Figure 3). A key observation was that adaptation occurs on a fine spatial scale before bipolar cell signals are pooled within the ganglion cell’s receptive field center (Figure 7). On the other hand, it must occur after convergence of bipolar cells and wide-field amacrine cells. There is only one circuit element that can meet these constraints: a synaptic terminal of a bipolar cell that receives inhibition from amacrine cells (site 5 in Figure 3). Thus our results suggest that the peripheral inhibition responsible for differential motion selectivity is, in large part, onto the presynaptic terminals of bipolar cells that provide excitation to the OMS ganglion cells.
It must be noted that only some bipolar cell terminals are affected by presynaptic inhibition from amacrine cells. For example, most ganglion cell types are not suppressed by Global Motion stimuli (Ölveczky et al., 2003). Similarly, the polyaxonal amacrine cells themselves are not suppressed; in fact, Global Motion is more effective than Differential Motion for these neurons (Figure 4B). Clearly, they must draw their input from a different set of bipolar cell terminals. Finally, the OMS ganglion cells themselves receive some input from non-suppressed bipolar cells, since they do show stimulus-evoked subthreshold voltage fluctuations during Global Motion (Figure 5B). Indeed, structural studies have shown that diverse bipolar cell types form different interactions with amacrine cells, and multiple bipolar types can converge on the same ganglion cell (Masland, 2001; McGuire et al., 1984).
Adaptation through presynaptic depression
On this background, adaptation in OMS ganglion cells can occur as follows: During Global Motion, the bipolar cells in the receptive field center depolarize synchronously with amacrine cells in the surround, whose axons inhibit the bipolar cell terminal. Thus the terminal releases no neurotransmitter and the OMS ganglion cell remains silent. During Differential Motion, bipolar cells in the center and amacrines in the surround depolarize asynchronously. Thus the transmission block is relieved and the OMS cells are excited. Continued activation of the bipolar cell terminal leads to presynaptic depression (von Gersdorff and Matthews, 1997; Burrone and Lagnado, 2000), a gradual decline in transmitter release, and thus a decline in OMS cell activity (Figure 1). During a subsequent period of Global Motion, the terminal is silenced again, and gradually recovers from depression (Figure 2).
The overall picture of OMS circuitry is summarized in Figure 3. Assuming that adaptation happens via synaptic depression at bipolar cell terminals (site 5 in Figure 3), one can explain all the observed phenomena: the lack of adaptation in amacrine cell signals (Figure 4); the decline in OMS cell inputs during Differential Motion (Figure 5); adaptation prior to spatial pooling in the receptive field surround (Figure 6); and adaptation prior to spatial pooling in the center (Figure 7). None of the alternative mechanisms considered here can account for all of these observations. Still, there could be other contributions to the overall effect. For example, a spike-dependent sodium conductance (Kim and Rieke, 2003) may alter the post-synaptic sensitivity of the OMS ganglion cell during periods of high activity. On the other hand, we found that the pre-synaptic component of adaptation is by itself strong enough to account for the two-fold effects of differential motion adaptation (Figures 1D–E, 6B, 7C).
The same mechanism could well be engaged in contrast adaptation. Following a sudden increase in the contrast of a stimulus, the ganglion cell gradually loses sensitivity (Smirnakis et al., 1997). This results in large part from a decline in synaptic input from bipolar cells (Kim and Rieke, 2001; Manookin and Demb, 2006), which could arise from activity-dependent depression of the bipolar cell terminal. Note, however, that an increase in contrast activates the bipolar terminal in a very different manner from differential motion: In the former case, the bipolar cell signal increases dramatically. In the latter case, the bipolar cell signal remains the same, but the terminal is relieved from amacrine inhibition.
Input-specific adaptation
We observed that different inputs to the same ganglion cell can adjust their relative weight if they are stimulated differentially (Figure 7): A continuously active bipolar cell terminal loses its voice, a silent one gains in strength. This dynamic flexibility of the retinal microcircuit may be part of a general strategy of predictive coding that serves to decrease the sensitivity to maintained features in the stimulus and enhance the response to novel features (Barlow, 1990). Specifically, each bipolar cell terminal could communicate a somewhat different aspect of the stimulus. It has its own receptive field, determined by excitation from the bipolar cell and inhibition from the particular amacrine cells that contact the terminal. Thus adaptation to this terminal’s preferred stimulus feature would occur independently of others. In fact, a surprisingly general form of pattern-specific adaptation has recently been observed in the retina (Hosoya et al., 2005), and it may well involve this same mechanism. Input-specific adaptation is also a well-known aspect of neural coding in the visual cortex (Movshon and Lennie, 1979), and indeed presynaptic depression has been invoked as a possible explanation (Chance et al., 1998). The phenomenon exists in other sensory systems (Best and Wilson, 2004; Gollisch and Herz, 2004), and likely represents an important motif in neural computation.
Experimental Procedures
Electrophysiology
Retinas of larval tiger salamanders were isolated in darkness and superfused with oxygenated Ringer’s medium at room temperature. A piece of retina, 6–8 mm on a side, was placed ganglion-cell-layer-down on a multi-electrode array, which recorded spike trains simultaneously from many ganglion cells, as described previously (Meister et al., 1994). For intracellular recordings (Baccus and Meister, 2002), sharp microelectrodes were filled with 2 M potassium acetate and 1 % Alexa 488, having a final impedance of 150–250 MΩ. Cells were identified by their responses to flashes, Differential and Global Motion stimuli, and by their depth within the retina. The recorded resting potentials were −55 to −65 mV for polyaxonal amacrine cells, −60 to −70 mV for OMS ganglion cells, and −45 to −60 mV for bipolar cells. Following recording, cells were filled with dye iontophoretically, and the cell type was confirmed by viewing the live preparation using a 40x water immersion objective.
Visual stimulation
Visual stimuli were projected from a computer monitor onto the photoreceptor layer, as described (Meister et al., 1994). All experiments used a mean photopic intensity of ~8 mW/m2. The jittered gratings consisted of black and white bars with a periodicity of 133 μm. The object region, 800 μm in diameter, was separated from the background region, measuring 4300 × 3200 μm, by a 67 μm grey annulus, except for the experiments in Figures 6 and 7, in which the annulus was 270 μm. For the experiments in Figures 1, 2, 4, 5, and 9 the jitter trajectory was generated by stepping the grating randomly in 1D every 15 ms with a step size of 6.7 μm. Each trial used a different random trajectory. On alternating trials, the trajectories in the object and background regions were either the same or different; all other aspects of the stimulus remained unchanged. For the experiments in Figures 4 and 5, the same motion trajectory was repeated, within a given trial, every 5 or 10 seconds; this was done to allow a better comparison of the subthreshold membrane potential fluctuations at different times relative to differential motion onset. For the experiments in Figures 6, 7, and 8 the grating motion was periodic. Speed of the grating was 450 μm/s; amplitude, and periodicity as stated in figure legends.
Analysis
The spatio-temporal receptive fields of all ganglion cells were measured by reverse correlation to a flickering black-and-white checkerboard stimulus (Meister et al., 1994). The spatio-temporal receptive field was approximated as the product of a spatial profile and a temporal filter. The receptive field center of a ganglion cell was estimated as the region where the spatial profile was larger than 1/3 of its maximum value. All polyaxonal amacrine cell recordings were from cells that were impaled in the object region of the stimulus.
Ganglion cells were classified on the basis of their spatio-temporal receptive fields (Warland et al., 1997). The Fast OFF ganglion cell (~60% of recorded cells) is the main ganglion cell type in the salamander showing differential motion selectivity (Ölveczky et al., 2003), and the analyses were done exclusively on these cells.
Only ganglion cells with receptive field centers enclosed by the object region were included in the analyses. Time constants quoted in the text and figures derive from the best exponential fit to the data. The standard deviation of intracellular membrane potentials referred to in the text and figures is the stimulus-driven component in excess of noise in the recording. The noise was estimated from the standard deviation in the recordings when no visual stimulus was present.
The cross-correlation function C(τ) in Figure 9 was calculated as
| (1) |
where r(t) is the instantaneous firing rate at time t, and 〈 … 〉 is averaged over 4-s intervals and over 100 trials using different motion trajectories. All cells were recorded in the stimulus configuration of Figure 1; cells 1 and 3 during the same trial; cell 2 during a trial with a different object trajectory.
Error bars in all figures denote standard error, derived from variation among cells or across trials. In averaging the relative firing rate across ganglion cells (Figures 4, 6, 7), each cell’s response was normalized to the steady-state firing rate in the differential motion condition during the final ~10 s of the stimulus.
Acknowledgments
We thank Dr Mihai Manu for help with bipolar cell recordings. This work was supported by the Harvard Society of Fellows (BPÖ), an NRSA postdoctoral fellowship (SAB) and grants from the NIH (MM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams RA, Christ SE. Motion onset captures attention. Psychol Sci. 2003;14:427–432. doi: 10.1111/1467-9280.01458. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Huang EJ, Manis PB, Linden DJ. Use-dependent changes in synaptic strength at the Purkinje cell to deep nuclear synapse. Prog Brain Res. 2000;124:257–273. doi: 10.1016/s0079-6123(00)24022-3. [DOI] [PubMed] [Google Scholar]
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. doi: 10.1016/s0896-6273(02)01050-4. [DOI] [PubMed] [Google Scholar]
- Barlow HB. A theory about the functional role and synaptic mechanism of visual after-effects. In: Blakemore C, editor. Vision: Coding and Efficiency. Cambridge: Cambridge University Press; 1990. pp. 363–375. [Google Scholar]
- Barlow HB, Hill RM. Evidence for a physiological explanation of the waterfall phenomenon and figural after-effects. Nature. 1963;200:1345–1347. doi: 10.1038/2001345a0. [DOI] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci. 2004;24:652–660. doi: 10.1523/JNEUROSCI.4220-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Masland RH. Spatial scale and cellular substrate of contrast adaptation by retinal ganglion cells. Nat Neurosci. 2001;4:44–51. doi: 10.1038/82888. [DOI] [PubMed] [Google Scholar]
- Burrone J, Lagnado L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci. 2000;20:568–578. doi: 10.1523/JNEUROSCI.20-02-00568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. J Neurosci. 1998;18:4785–4799. doi: 10.1523/JNEUROSCI.18-12-04785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Clifford CW, Ibbotson MR. Fundamental mechanisms of visual motion detection: models, cells and functions. Prog Neurobiol. 2002;68:409–437. doi: 10.1016/s0301-0082(02)00154-5. [DOI] [PubMed] [Google Scholar]
- Cook PB, Lukasiewicz PD, McReynolds JS. Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. J Neurosci. 1998;18:2301–2308. doi: 10.1523/JNEUROSCI.18-06-02301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeese MR, Zador AM. Asymmetric dynamics in optimal variance adaptation. Neural Computation. 1998;10:1179–1202. [Google Scholar]
- Engbert R, Kliegl R. Microsaccades keep the eyes’ balance during fixation. Psychol Sci. 2004;15:431–436. doi: 10.1111/j.0956-7976.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Shapley RM. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973;233:271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaschi D, Douglas R, Marlin S, Cynader M. The time course of direction-selective adaptation in simple and complex cells in cat striate cortex. J Neurophysiol. 1993;70:2024–2034. doi: 10.1152/jn.1993.70.5.2024. [DOI] [PubMed] [Google Scholar]
- Gollisch T, Herz AV. Input-driven components of spike-frequency adaptation can be unmasked in vivo. J Neurosci. 2004;24:7435–7444. doi: 10.1523/JNEUROSCI.0398-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare WA, Owen WG. Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J Neurophysiol. 1996;76:2005–2019. doi: 10.1152/jn.1996.76.3.2005. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Dorn TJ, Bach M. Time course of motion adaptation: motion-onset visual evoked potentials and subjective estimates. Vision Res. 1999;39:437–444. doi: 10.1016/s0042-6989(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature. 2005;436:71–77. doi: 10.1038/nature03689. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Rieke F. Slow Na+ inactivation and variance adaptation in salamander retinal ganglion cells. J Neurosci. 2003;23:1506–1516. doi: 10.1523/JNEUROSCI.23-04-01506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Rieke F. Temporal contrast adaptation in the input and output signals of salamander retinal ganglion cells. J Neurosci. 2001;21:287–299. doi: 10.1523/JNEUROSCI.21-01-00287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Poggio T, Torre V. Nonlinear interactions in a dendritic tree: localization, timing, and role in information processing. Proc Natl Acad Sci U S A. 1983;80:2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E. Eye Movements and Their Role in Visual and Cognitive Processes. New York: Elsevier; 1990. [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Demb JB. Presynaptic mechanism for slow contrast adaptation in mammalian retinal ganglion cells. Neuron. 2006;50:453–464. doi: 10.1016/j.neuron.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Manteuffel G, Plasa L, Sommer TJ, Wess O. Involuntary eye movements in salamanders. Naturwissenschaften. 1977;64:533–534. doi: 10.1007/BF00483559. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Pine J, Baylor DA. Multi-neuronal signals from the retina: acquisition and analysis. J Neurosci Methods. 1994;51:95–106. doi: 10.1016/0165-0270(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature. 1979;278:850–852. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- Ölveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–408. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- Rieke F. Temporal contrast adaptation in salamander bipolar cells. J Neurosci. 2001;21:9445–9454. doi: 10.1523/JNEUROSCI.21-23-09445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000;20:4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skavenski AA, Hansen RM, Steinman RM, Winterson BJ. Quality of retinal image stabilization during small natural and artificial body rotations in man. Vision Research. 1979;19:675–683. doi: 10.1016/0042-6989(79)90243-8. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Berry MJ, Warland DK, Bialek W, Meister M. Adaptation of retinal processing to image contrast and spatial scale. Nature. 1997;386:69–73. doi: 10.1038/386069a0. [DOI] [PubMed] [Google Scholar]
- Sugase Y, Yamane S, Ueno S, Kawano K. Global and fine information coded by single neurons in the temporal visual cortex. Nature. 1999;400:869–873. doi: 10.1038/23703. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Van Wezel RJ, Britten KH. Motion adaptation in area MT. J Neurophysiol. 2002;88:3469–3476. doi: 10.1152/jn.00276.2002. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci. 1997;17:1919–1927. doi: 10.1523/JNEUROSCI.17-06-01919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu ET, Krasne FB. Evidence for a computational distinction between proximal and distal neuronal inhibition. Science. 1992;255:1710–1712. doi: 10.1126/science.1553559. [DOI] [PubMed] [Google Scholar]
- Warland DK, Reinagel P, Meister M. Decoding visual information from a population of retinal ganglion cells. J Neurophysiol. 1997;78:2336–2350. doi: 10.1152/jn.1997.78.5.2336. [DOI] [PubMed] [Google Scholar]