Abstract
Amphetamine-induced motor behaviors, i.e., locomotor and stereotypic activities, were simultaneously characterized in C57BL/6 mice, a strain commonly used for genetic studies. Our findings show relatively high levels of focused activities in drug-naïve C57BL/6 mice, confirming the lively nature of this mouse strain. Acute amphetamine induced a dose-dependent, bimodal response: locomotion predominated at lower doses of amphetamine and was gradually displaced by stereotypic behavior as dose and time increased. The sum total of both behavioral activities increased with amphetamine dose, supporting the notion that amphetamine-induced locomotion and stereotypy form a continuum. These data provide a basis for using C57BL/6 mice as a strain to study the molecular and cellular mechanisms underlying psychostimulant effects, drug addiction and psychotic disorders.
Keywords: amphetamine, C57BL/6, locomotion, stereotypy, motor behavior, mouse
Introduction
Amphetamine and other drugs of abuse are known to act on the “brain reward circuitry”, a target they have in common with natural stimuli such as food and sex [9]. The two main pathways involved are the mesolimbic and nigrostriatal dopamine systems: neurons projecting from the ventral tegmental area (VTA) and substantia nigra (SN) to the nucleus accumbens (NAc) and dorsal striatum, respectively [7]. Although their cellular mechanisms are not the same, all drugs of abuse share the property that they increase extracellular dopamine (DA) levels, mostly in the NAc, and enhance motor activity [24]. In fact, the motor-stimulant potency of a drug is considered a measure for its reinforcing, addictive properties [11]. Moreover, overactivation of DA neurotransmission is one characteristic that drug-induced behaviors may have in common with psychotic disorders [19,21].
Animal models of amphetamine-induced motor activation are regularly used in studying the molecular and cellular mechanisms underlying drug addiction and psychiatric disorders. The drug-induced state can either model the changes in these disorders or serve as a means to evaluate the effects of pharmacological or genetic manipulation. Much of our knowledge on the effects of amphetamine is based on characterization in the rat, since past studies generally used this animal. Dose-response tests with amphetamine indicated a bell-shaped locomotor response, whereby low doses augment locomotor behavior, while higher doses enhanced “competing” focused stereotypic movements [1,10,17]. Genetically engineered animal models include primarily mice. To properly evaluate these models for studying mechanisms underlying psychostimulant effects, drug addiction and psychotic disorders, a thorough characterization of the effects of drugs of abuse in mice is essential. Therefore, the present study was undertaken to examine both amphetamine-induced locomotor and stereotypy activity using C57BL/6 mice, a strain commonly used in gene manipulation studies.
Materials and Methods
Animals
Male C57BL/6 mice (Charles River Laboratories, Raleigh, NC), 5 to 7 weeks old (18–25g), were housed 4 per cage with food and water available ad libitum, and maintained on a 12 h light: 12 h dark cycle. At least 24 hours before behavioral testing, mice were moved to the testing facility to acclimate. Each mouse was naïve to the drugs and was used only one time. All experimental protocols complied with the NIH Guide for the care and use of laboratory animals and approved by the University of Cincinnati IACUC.
Drugs
d-Amphetamine was obtained from the National Institute on Drug Abuse (NIDA) and dissolved in saline (0.9% NaCl). Saline without drug was used as vehicle control. The drug weights used were of the salt form and were not corrected for the free base. All injections were subcutaneous in a final volume of 1 ml/kg.
Locomotor and Stereotypic Activity
Behavioral characterization was carried out in custom-designed residential activity chambers (RACs) as we previously described [20], modeled after the design of Segal and Kuczenski [22]. Mice were randomly assigned to a RAC and allowed to habituate for at least 30 minutes, then weighed, injected subcutaneously with the drug or vehicle, and promptly placed back, whereupon recording of locomotion and video taping were started. Locomotor activity was expressed as crossovers, defined as the number of times the animal entered into any of 5 zones (4 corners and 1 center) out of the 9 equally-sized ones subdividing the enclosure as we previously described [20]. The photobeam break data were collected by computer using Flex Field software (San Diego Instruments). Stereotypy was coded during off-line videotape viewing by two observers independently, who were blind to the treatment conditions of each mouse. Each observer measured the cumulative time a mouse spent engaged in stereotypy for the first 2 minutes of every 5 minute interval in the 2 hour test period. Stereotypy was defined as focused engagement in repetitive head and limb movements in a stationary posture; therefore, focused repetition of “normal” head and limb movements, such as grooming, licking, sniffing, etc., were included in the scoring (see also Discussion).
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA) by ANOVA followed by Bonferroni’s post hoc tests, as indicated in the Figure Legends. Significance was set at p < 0.05.
Results
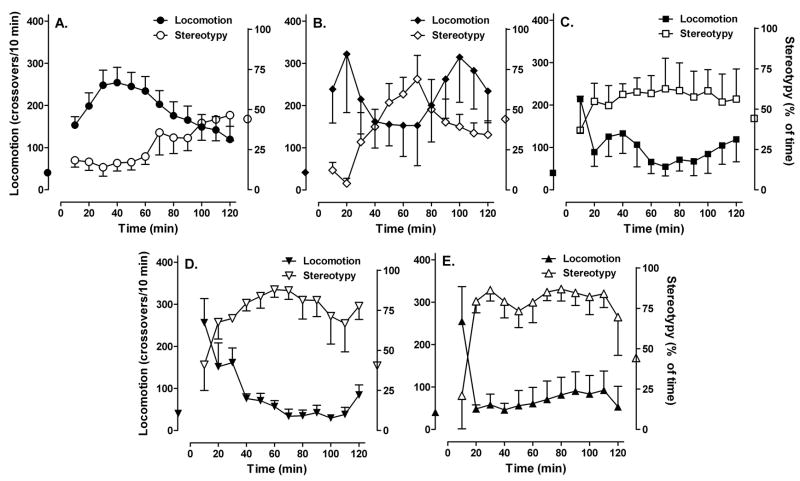
Treatment of rodents with amphetamine is known to cause an increase in motor behaviors. We used C57BL/6 mice, a strain commonly used for genetic studies, and examined the levels of horizontal locomotion (ambulation) and stereotyped movements. We wished to examine a range of amphetamine doses, in particular to compare and contrast lower doses with higher ones. Therefore we chose 2 doses each in the lower range (2 and 6 mg/kg) and the higher range (16 and 20 mg/kg), plus an intermediate dose (12 mg/kg). Amphetamine at 2 mg/kg resulted in a robust locomotor response that reached peak values between 30 and 60 min after injection (Fig. 1A). At the same time, baseline focused movements were reduced (Fig. 1A). After 6 mg/kg amphetamine, locomotion was further increased but interrupted by a period of stereotypic behaviors (Fig. 1B). An early peak in hyperlocomotion was also observed after 12 mg/kg and higher doses of amphetamine, but that was rapidly displaced by stereotypic behaviors for the remainder duration (Fig. 1C,D,E). Time spent in stereotypy reached a significant elevation above baseline after 16 and 20 mg/kg (Fig. 1D,E). Amphetamine induced a biphasic effect in locomotion, with an initial peak of locomotion activity followed by suppression. As the amphetamine doses were increased, the initial peak of locomotion activity became less pronounced, while the locomotion suppression became more prolonged. At all doses, total activity reached a peak between 30 and 60 min after amphetamine injection, albeit that the full duration of the amphetamine effect increased with higher doses. A downgrading from stereotypy to hyperlocomotion was noted 80 to 100 min after 6 mg/kg, whereas this presumably occurred beyond the recording time after 12, 16 and 20 mg/kg amphetamine. Cumulative locomotor activity during the 30–60 min peak period was significantly increased after 2 mg/kg amphetamine, and gradually declined after higher doses of amphetamine were given (Fig. 2A). After 12 mg/kg, the increase was still well above baseline but also significantly decreased as compared to 2 mg/kg. Conversely, total stereotypic behavior in this 30–60 min period was significantly suppressed after 2 mg/kg amphetamine, and stereotypy gradually rose to reach a significant increase above baseline after 16 and 20 mg/kg amphetamine (Fig. 2B).
Fig. 1.
Time courses of amphetamine-induced motor behaviors. Mice were injected s.c. with (A) 2 mg/kg (n=8), (B) 6 mg/kg (n=4), (C) 12 mg/kg (n=7), (D) 16 mg/kg (n=5) or (E) 20 mg/kg d-amphetamine (n=4) or saline (n=11). Locomotion and stereotypy were recorded simultaneously. Data are mean (± SEM) number of crossovers (closed symbols, left Y-axis) and percent time in stereotypy (open symbols, right Y-axis), respectively, in each preceding 10 min interval. Mean values after Saline are indicated on the left (locomotion) and right (stereotypy) Y axes. Two-way rmANOVA detected significant differences at particular time points (not indicated), in locomotor activity after 2 and 6 mg/kg and in stereotypy after 2, 16 and 20 mg/kg amphetamine.
Fig. 2.
Dose-response of amphetamine-induced motor behaviors. Shown are mean (+ SEM) cumulative locomotor activity (A) and percent time in stereotypy (B) in the period from 30 to 60 min after injection. *p < 0.05 vs. Saline; #p < 0.05 vs. 2 mg/kg amphetamine (one-way ANOVA with Bonferroni posttests).
For a more direct dose-related comparison of the levels of locomotor and stereotypic behaviors, we sought to express locomotion as percent of maximum activity. The highest activity measured in an individual mouse in our study, was 777 crossovers per 10 min interval. Normalized to this value, the highest average value of locomotor activity was 41.4%. Total locomotor activities over the 2 h recording period showed a general trend of decrease as amphetamine doses increased (Fig. 3, solid circles), similar to amphetamine dose-dependence over the peak 30–60 min period described above. For total stereotypic behavior, a general trend of increase was evident (Fig. 3, open circles), again similar to amphetamine effect over the peak 30–60 min period described above. The sum total of locomotor and stereotypic activities indicated that the total motor activity rose with increasing amphetamine dosage, displaying a curvilinear relationship (Fig. 3, dotted line). This supports the notion that amphetamine-induced stereotypy and locomotor behavior form a continuum of psychomotor activities.
Fig. 3.
Inverse relationship of amphetamine-induced locomotor and stereotypic behaviors. Shown are percent of maximum crossovers (closed circles) and percent time in stereotypy (open circles) for 2 h after injection. The sum total of the two activities (stars) displays a curvilinear relationship (dotted line, R2=0.90).
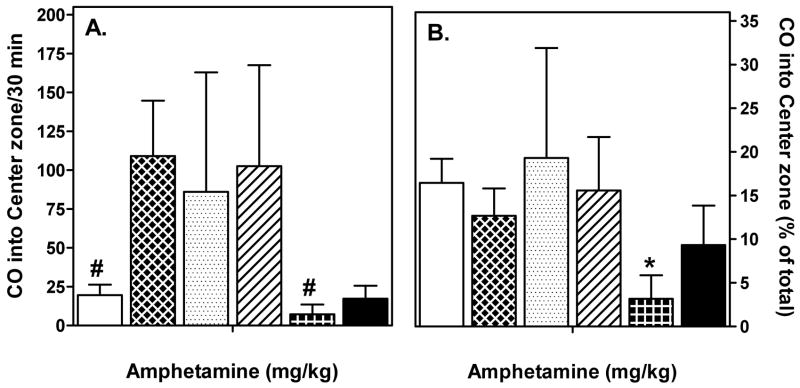
It has been observed in rats that amphetamine-induced locomotion can have a stereotyped pattern, specifically, a movement back and forth along one side of the cage [19,22]. To determine whether the horizontal locomotor pattern we observed in C57BL/6 mice was stereotyped, crossovers into the virtual central and 4 peripheral zones of the arena were derived separately. Over the 30–60 min peak period, the number of crossovers into the center tended to increase after 2, 6, and 12 mg/kg (Fig. 4A) more or less proportional to the total number of crossovers (compare Fig. 2A). Moreover, when evaluated as percent crossovers into the center per mouse, a significant decrease after 16 mg/kg was observed (Fig. 4B). Thus, in general, the mice did not show a preference for peripheral zones during amphetamine-induced locomotion. However, mice did move relatively closer to the wall during a period that also included significant focused stereotypic behavior.
Fig. 4.
Number of entries into the center zone after amphetamine administration. Mice received the indicated dose of amphetamine s.c. Locomotion was recorded as described under Methods and data for center zone were extracted. Data are mean (+ SEM) number of crossovers (A) and percent of total crossovers (B) in the period from 30 to 60 min after injection. *p < 0.05 vs. Saline; #p < 0.05 vs. 2 mg/kg amphetamine (one-way ANOVA with Bonferroni posttests).
Discussion
The present study characterized the behavioral responses to amphetamine in the C57BL/6, a widely used mouse strain, particularly genetic studies. In order to determine the effect of a modified gene on a particular drug response, it is essential to examine all behaviors over the time course of action of the drug [19]. However, there is still a lack of a characterization of the psychomotor behaviors over the time course of action of the drug amphetamine in C57BL/6 mice. Therefore, we set out to simultaneously record amphetamine-induced locomotor activity and stereotypy over the course of 2 hours, over a range of amphetamine doses. Locomotor activity can be readily instrument-recorded; amphetamine-induced stereotypy, however, merits further consideration in the mouse.
Stereotypy is not a well-defined behavioral parameter. Rather, it expresses the repetitive, persistent nature of otherwise normal activities. Hence, various methods to measure stereotypy have been described in the literature. For example, the behavioral rating scales of Creese & Iversen (1973) [5] and Ellinwood & Balster (1974) [8] are often used; however, these scales were designed for rats. Moreover, these rating scales include increased locomotion as an intermediate form of stereotyped behavior. In addition, the use of rating scales has become contentious because of advances in automated recording. Specifically, tracking animal by photobeam arrays enables quantification of locomotor activity on a continuous scale [17,22], and limiting stereotypy to focused movements while the animal is stationary [2,17]. Thus, to quantify stereotypy on a continuous scale, we measured the duration of stereotypic behavior per time interval, as described by Segal & Kuzcenski [22]. This method to measure levels of both locomotion and stereotypy allows evaluation of the two behaviors independently and in relation to each other, thus providing a comprehensive profile of the behavioral response to amphetamine. Moreover, it provides a unified quantitative scale that may facilitate comparison among different studies.
Behaviors included in our stereotypy scoring were those described in the above-mentioned behavioral rating scales of rats [5,8]. Naïve and saline-injected animals can also display most of these behaviors [19]. Indeed, our observers, blind to treatment, could often not distinguish between certain normal behaviors that were “stationary” [5] or “inplace” [8] vs. drug-induced behaviors that were “in one location” [5] or “restricted” [8]. Since we wanted to provide a comprehensive description of amphetamine-induced behaviors in C57BL/6 mice, it would be inappropriate to exclude any behaviors that may be affected by psychostimulants. Interestingly, in contrast to most reports, in our study the saline-injected animals displayed focused repetitive movements for close to 50% of the time (Fig. 1, right Y axes; Fig. 2B, open column). This level of basal activity is consistent with behavioral counts in C57BL/6 mice reported by Nally et al. [18]. The high basal level of focused behavior allowed observation of its reduction upon amphetamine-induced stimulation of locomotion (Fig. 1A and B), without masking the robust increase in stereotypy after higher doses of amphetamine (Fig. 1D,E; Fig. 2). The presentation of data in Figs. 1 and 2, in different units on separate Y axes, may give the impression that locomotion and stereotypy are opposite, competing behaviors. To determine whether this was the case, we designed a method to transform the two sets of activity data to the same unit of measure — percent of maximum. Stereotyped behavior was already in this format, because 100% was the entire time interval. Since the locomotor activity does not have an obvious upper limit, we chose the maximum activity we observed in a mouse (777 crossovers per 10 min) to normalize the data. Mouse locomotion has a darting pattern [13], so the number of crossovers reflects time spent moving rather than individual speed. With this approach to calculate activity, our results show that the average peak in stereotypy is around 80%, whereas locomotion maxes out at circa 35% (Fig. 3). This is an indication that stereotypy represents a more intense form of motor activity. Moreover, this transformation allowed us to add the activities. The sum total activity of psychomotor behavior rose with increasing amphetamine dosage, whereby locomotion predominated at lower doses and stereotypy predominated at higher doses.
It should be emphasized that there is no clear distinction between the two: stereotypic behaviors were often displayed by animals while in locomotion, whereas the locomotion pattern was stereotyped in that mice moved more in the periphery of the arena at amphetamine doses that induced significant focused stereotypy (Fig. 4). Therefore, our data are consistent with the notion that stereotypy is an extension to, or a more intense “patterned” form of, locomotion, and that these behaviors form a continuum of psychomotor activities [19,22].
The notion that stereotypy is an extension of locomotor stimulation is also supported by the phenomenon of behavioral sensitization [15]. A dose of amphetamine that induces locomotion after a first application, yields enhanced locomotion progressing to stereotypy upon repeated applications or longer withdrawal periods. This does not preclude the possibility that amphetamine-induced stereotypy and hyperlocomotor are mediated by separate neural systems. Early studies in the rat showed that amphetamine-induced stereotypy is blocked by nigrostriatal lesioning, whereas its locomotor activation is absent in NAc-lesioned animals [6,11,14]. Additionally, in vivo microdialysis studies showed that amphetamine-induced locomotion and stereotypy are mediated via dopamine released in NAc and striatum, respectively [16,23]. However, the lack of correlation between the duration of increased dopamine levels and of behavior has been harder to interpret [4]. Interestingly, boosting striatal dopamine content in dopamine-deficient mice to levels that induced either locomotion or stereotypy, resulted in c-fos activation in (ventro)lateral striatum but not in NAc [3]. In contrast, when dopamine synthesis was rescued in these mice by viral vector expressing tyrosine hydroxylase, injection into only NAc restored locomotor responses to psychostimulant [12]. The present study may provide a basis for future studies on psychostimulant-induced behaviors by pharmacological and genetic manipulations.
Conclusion
This study characterized amphetamine-induced locomotion and stereotypy in C57BL/6 mice. Results suggested that the two behaviors may be considered as a continuum of the animal’s psychomotor activity. The relatively high level of focused activity of this mouse strain provides a sensitive endpoint to observe either up- or down-regulation by pharmacological and genetic manipulations. Thus, this study demonstrated the favorability of using this strain of mice as a model to examine the molecular and cellular mechanisms underlying psychostimulant effects, drug addiction and psychiatric disorders involving altered psychomotor behavior.
Acknowledgments
This work was supported by the Department of Veterans Affairs Medical Research Service (NMR) and the National Institute of Drug Abuse (DA0599101 (JY), DA016778 (NMR), DA013471 and DA020555 (LY)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–196. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- 2.Battisti JJ, Uretsky NJ, Wallace LJ. NMDA glutamate receptor role in the development of context-dependent and independent sensitization of the induction of stereotypy by amphetamine or apomorphine. Behav Brain Res. 2000;114:167–174. doi: 10.1016/s0166-4328(00)00227-8. [DOI] [PubMed] [Google Scholar]
- 3.Chartoff EH, Marck BT, Matsumoto AM, Dorsa DM, Palmiter RD. Induction of stereotypy in dopamine-deficient mice requires striatal D1 receptor activation. PNAS. 2001;98:10451–10456. doi: 10.1073/pnas.181356498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti LH, Segal DS, Kuczenski R. Maintenance of amphetamine-induced stereotypy and locomotion requires ongoing dopamine receptor activation. Psychopharmacology (Berl) 1997;130:183–188. doi: 10.1007/s002130050227. [DOI] [PubMed] [Google Scholar]
- 5.Creese I, Iversen SD. Blockage of amphetamine induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- 6.Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- 7.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellinwood EH, Jr, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- 9.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaytan O, Swann A, Dafny N. Time-dependent differences in the rat’s motor response to amphetamine. Pharmacol Biochem Behav. 1998;59:459–467. doi: 10.1016/s0091-3057(97)00438-3. [DOI] [PubMed] [Google Scholar]
- 11.Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr. 1989;94:101–126. [PubMed] [Google Scholar]
- 12.Heusner CL, Hnasko TS, Szczypka MS, Liu Y, During MJ, Palmiter RD. Viral restoration of dopamine to the nucleus accumbens is sufficient to induce a locomotor response to amphetamine. Brain Res. 2003;980:266–274. doi: 10.1016/s0006-8993(03)02986-x. [DOI] [PubMed] [Google Scholar]
- 13.Kafkafi N, Pagis M, Lipkind D, Mayo CL, Bemjamini Y, Golani I, Elmer GI. Darting behavior: a quantitative movement pattern designed for discrimination and replicability in mouse locomotor behavior. Behav Brain Res. 2003;142:193–205. doi: 10.1016/s0166-4328(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 14.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 15.Kilbey MM, Ellinwood EH., Jr Reverse tolerance to stimulant-induced abnormal behavior. Life Sciences. 1977;20:1063–1075. doi: 10.1016/0024-3205(77)90294-6. [DOI] [PubMed] [Google Scholar]
- 16.Kuczenski R, Melega WP, Cho AK, Segal DS. Extracellular dopamine and amphetamine after systemic amphetamine administration: comparison to the behavioral response. J Pharmacol Exp Ther. 1997;282:591–596. [PubMed] [Google Scholar]
- 17.Kuczenski R, Segal D. Concomitant Characterization of Behavioral and Striatal Neurotransmitter Response to Amphetamine Using Invivo Microdialysis. Journal of Neuroscience. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nally RE, Kinsella A, Tighe O, Croke DT, Fienberg AA, Greengard P, Waddington JL. Ethologically Based Resolution of D2-Like Dopamine Receptor Agonist-versus Antagonist-Induced Behavioral Topography in Dopamine- and Adenosine 3′,5′-Monophosphate-Regulated Phosphoprotein of 32 kDa “Knockout” Mutants Congenic on the C57BL/6 Genetic Background. J Pharmacol Exper Ther. 2004;310:1281–1287. doi: 10.1124/jpet.104.068957. [DOI] [PubMed] [Google Scholar]
- 19.Rebec GV, Bashore TR. Critical issues in assessing the behavioral effects of amphetamine. Neurosci Biobehav Rev. 1984;8:153–159. doi: 10.1016/0149-7634(84)90030-7. [DOI] [PubMed] [Google Scholar]
- 20.Richtand NM, Logue AD, Welge JA, Perdiue J, Tubbs LJ, Spitzer RH, Sethuraman G, Geracioti TD. The dopamine D3 receptor antagonist nafadotride inhibits development of locomotor sensitization to amphetamine. Brain Res. 2000;867:239–242. doi: 10.1016/s0006-8993(00)02247-2. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 22.Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- 23.Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF. The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav. 1986;25:233–248. doi: 10.1016/0091-3057(86)90261-3. [DOI] [PubMed] [Google Scholar]