Abstract
In humans, exposure to environmental contexts previously associated with heroin intake can provoke drug relapse, but the neuronal mechanisms mediating this relapse are unknown. Using a drug relapse model, we found previously that reexposing rats to heroin-associated contexts, after extinction of drug-reinforced responding in different contexts, reinstates heroin seeking. This effect is attenuated by inhibition of glutamate transmission in the ventral tegmental area and medial accumbens shell, components of the mesolimbic dopamine system. Here, we explored the role of dopamine of the accumbens in context-induced reinstatement by using the D1-family receptor antagonist SCH 23390 [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride]. Rats were trained to self-administer heroin for 12 d; drug infusions were paired with a discrete tone–light cue. Subsequently, the heroin-reinforced lever pressing was extinguished in the presence of the discrete cue in a context that differed from the drug self-administration context in terms of visual, auditory, tactile, and circadian cues. When tested in the original drug self-administration context, systemic and medial or lateral accumbens shell SCH 23390 injections attenuated context-induced reinstatement of heroin seeking, whereas accumbens core SCH 23390 injections were ineffective. In contrast, core but not lateral or medial shell SCH 23390 injections attenuated discrete-cue-induced reinstatement in a nondrug context after extinction of lever presses without this cue. Results indicate that activation of medial and lateral accumbens shell D1-family dopamine receptors mediate context-induced reinstatement of heroin seeking and provide the first demonstration for a role of lateral shell dopamine in conditioned drug effects. Results also demonstrate novel dissociable roles of accumbens core and shell in context- versus discrete-cue-induced reinstatement of heroin seeking.
Keywords: conditioned cues, drug abuse, nucleus accumbens, opiates, reinstatement, relapse, SCH 23390
Introduction
Environments previously associated with opiate intake can provoke drug relapse during abstinence (Wikler, 1973; O'Brien et al., 1992). Despite this evidence, the role of contextual stimuli (e.g., the physical characteristics of drug environment, time of day) in preclinical drug relapse models has until recently been ignored (Shalev et al., 2002; Bossert et al., 2005b). This issue is important for understanding drug relapse, because environmental contexts strongly influence extinction and resumption of learned behaviors (Bouton, 2002). We adapted a renewal procedure (Bouton and Bolles, 1979) to study the role of environmental contexts in reinstatement of drug seeking (Crombag et al., 2002; Crombag and Shaham, 2002). Using variations of this procedure, we and others found that reexposing rats to drug-associated contexts after extinction of drug-reinforced responding in different contexts reinstates heroin, cocaine, and alcohol seeking (Bossert et al., 2004; Fuchs et al., 2005; Burattini et al., 2006; Fletcher et al., 2007).
Using the renewal procedure, we identified a role for glutamate transmission in mesolimbic areas in context-induced reinstatement of heroin seeking. Injections of LY379268 (1R,4R,5S,6R-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate) [group II metabotropic glutamate receptors (mGluR2 and 3) agonist that decreases evoked glutamate release] into the ventral tegmental area (VTA) or medial accumbens shell attenuated this reinstatement (Bossert et al., 2004, 2006). The VTA and accumbens shell are the respective cell body and terminal regions of the mesolimbic dopamine system (Fallon and Moore, 1978). Thus, our findings suggest that the role of glutamate in context-induced reinstatement involves modulation of mesolimbic dopamine function. Consistent with this hypothesis, systemic injections of D1-family receptor antagonists (SCH 23390 [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride] and SCH 39166 [(−)-trans-6,7,7a,8,9,13b-hexahydro-3-chloro-2-hydroxy-N-methyl-5a-benzo-(D)-naphtho-(2,1b) azepine]) decrease context- and discriminative-cue-induced reinstatement of cocaine, alcohol, and sucrose seeking (Ciccocioppo et al., 2001; Weiss et al., 2001; Crombag et al., 2002; Hamlin et al., 2006, 2007). Also, systemic SCH 23390 injections decrease context-induced increases in Fos expression in accumbens shell (Hamlin et al., 2006, 2007).
Based on these studies, we first examined the effect of systemic and medial accumbens shell injections of SCH 23390 on context-induced reinstatement of heroin seeking. SCH 23390 rapidly diffuses away from its injection site (Caine et al., 1995), and there is evidence for a role of accumbens core in conditioned drug effects (Everitt and Robbins, 2005). Thus, we also tested the effect of accumbens core SCH 23390 injections on context-induced reinstatement. There are anatomical differences between medial and lateral accumbens shell in both neuronal morphology and connectivity (Meredith et al., 1992; Voorn et al., 2004; Ikemoto, 2007), and these subregions play different roles in drug (Ikemoto, 2003; Ikemoto et al., 2005) and food (Basso and Kelley, 1999; Zhang and Kelley, 2000) reward. Thus, we also assessed the effect of lateral accumbens shell SCH 23390 injections on context-induced reinstatement. Finally, to further assess the role of the accumbens in reinstatement induced by heroin cues, we examined the effect of SCH 23390 injections into the medial shell, lateral shell, and core on discrete-cue-induced reinstatement of heroin seeking. In this procedure, reinstatement of drug seeking induced by contingent exposure to a compound tone–light cue (previously paired with drug infusions during training) is assessed after extinction of lever presses without this cue (See, 2002).
Materials and Methods
Subjects.
Male Long–Evans rats (total n = 273; Charles River Laboratories, Raleigh, NC), weighing 350–450 g were used. After surgery, the rats were housed individually in the animal facility under a reverse 12 h light/dark cycle (lights off at 9:00 A.M.). Food and water were available ad libitum in the rats' home cage throughout the experiment. Experimental procedures followed the guidelines of the Principles of Laboratory Animal Care (National Institutes of Health publication number 86-23, 1996). Seventy-nine rats were excluded because of catheter problems, failure to learn to self-administer heroin, poor health, misplaced or blocked cannulas, or failure to meet an extinction criterion of 25 responses per 3 h.
Intracranial and intravenous surgery.
Rats were anesthetized with sodium pentobarbital and chloral hydrate (60 and 25 mg/kg, i.p.), and permanent guide cannulas (23 gauge; Plastics One, Roanoke, VA) were implanted bilaterally 1 mm dorsal to the medial accumbens shell, lateral accumbens shell, or accumbens core, using stereotaxic coordinates (Paxinos and Watson, 2005) that are based on our previous work (Bossert et al., 2006) and the work of Ikemoto (2003, 2007). The coordinates for the core (6° angle) were as follows: anteroposterior (AP), +1.7 mm; mediolateral (ML), ±2.5 mm; dorsoventral (DV), −6.0 mm. The coordinates for the lateral shell (4° angle) were as follows: AP, +2.0 mm; ML, ±2.6 mm; DV, −7.2 mm. The coordinates for the medial shell were either of the following: AP, +1.7 mm; ML, ±1.6; DV, −6.5 mm (6° angle); or AP, +1.7 mm; ML, ±3.6 mm; DV, −7.2 mm (25° angle). Note that, in initial runs in experiment 2, we used the 6° angle coordinates for the medial shell. However, because the cannulas passed through the ventricles, we performed angiotensin (12.5 ng/side)-induced drinking tests (Johnson and Epstein, 1975) in drug-naive rats that did not take part in the behavioral experiments. These rats were implanted with bilateral cannulas into the medial or lateral accumbens shell (n = 10 per brain site). Our findings from this experiment suggested that medial shell (but not lateral shell) SCH 23390 injections can diffuse into the ventricles in approximately half of the rats. Thus, we adjusted our coordinates to assess the effect of medial shell SCH 23390 (0.6 μg/side, n = 4) or vehicle (n = 4) injections on context-induced reinstatement. We found that, with these new coordinates, SCH 23390 robustly decreased context-induced reinstatement (mean ± SEM of 67 ± 23 vs 8 ± 5 active lever presses per 3 h). On completion of this experiment, we injected these rats with 12.5 ng/side angiotensin and did not observe angiotensin-induced drinking. The data of these rats were combined with the previous runs, and the new 25° angle coordinates were then used for all rats in experiment 3 (discrete-cue-induced reinstatement).
After cannula implantation, silastic catheters were inserted into the jugular vein, as described previously (Shaham et al., 1996; Shalev et al., 2001). The catheters were attached to a modified 22 gauge cannula and mounted to the rats' skull with dental cement. Buprenorphine (0.1 mg/kg, s.c.) was given after surgery, and rats were allowed to recover for 7–10 d before behavioral testing began. During the recovery and training phases, catheters were flushed every 24–48 h with gentamicin (0.08 mg/ml) and sterile saline.
Systemic and intracranial injections.
SCH 23390 hydrochloride (Tocris Bioscience, Ellisville, MO) was dissolved in sterile saline. Systemic injections (2.5 or 5.0 μg/kg, s.c.) were given 15 min before the test sessions; doses are based on our previous report (Crombag et al., 2002). Bilateral intracranial injections (0.3 or 0.6 μg/side) were given 5–10 min before testing. These doses are lower than those typically used in studies in which SCH 23390 was injected intracranially to examine its effects on drug reward and reinstatement (Hurd et al., 1997; Ranaldi and Wise, 2001; Anderson et al., 2003; Alleweireldt et al., 2006), because SCH 23390 rapidly diffuses away from its injection site (Caine et al., 1995). Intracranial injections were made using a syringe pump (Harvard Apparatus, Holliston, MA) connected to 10 μl Hamilton syringes that were attached via polyethylene-50 tubing to 30 gauge injectors. SCH 23390 or vehicle (0.3 μl) injections were made over 1 min, and the injectors were left in place for 1 min. After testing, the rats were deeply anesthetized, then they were decapitated, and the brains were removed. Coronal sections (40 μm) were sliced on a cryostat and stained with cresyl violet. The brains were then verified for cannula placement under a light microscope.
Apparatus.
The rats were trained and tested in standard Med Associates (St. Albans, VT) operant chambers. Each chamber was equipped with two levers located 9 cm above the grid floor. Lever presses on the active retractable lever activated the infusion pump, whereas lever presses on the inactive nonretractable lever had no programmed consequences. The two contexts differed from each other in their auditory, visual, tactile, and circadian [i.e., morning (session onset at 9:00 A.M.) vs afternoon (session onset at 3:00 P.M.) sessions] cues using procedures identical to those described by Bossert et al. (2006). The contexts are referred to as A and B, whereby A is the self-administration context and B is the extinction context. The physical environments that provided contexts A and B and circadian cues were counterbalanced.
Experiments 1 and 2: effect of systemic and accumbens SCH 23390 injections on context-induced reinstatement.
The experimental procedure consisted of three phases: self-administration training (12 d), extinction training (14–25 d), and tests for context-induced reinstatement of heroin seeking (2 d). The contexts are labeled as context A (heroin self-administration context) and context B (non-heroin extinction context) and the experimental sequence was A (training)–B (extinction)–A (testing).
Heroin self-administration training and extinction.
Rats were trained to self-administer heroin for 3-h/d for 12 d. Heroin (diacetylmorphine HCl; National Institute on Drug Abuse, Baltimore, MD) was dissolved in sterile saline and infused at a volume of 65 μl over 2.3 s at a dose of 0.1 (first six sessions) and 0.05 (last six sessions) mg/kg per infusion. During training, heroin infusions were earned under a fixed ratio 1 (FR1), 2.3 s timeout reinforcement schedule and were accompanied by a compound tone–light cue for 2.3 s. During the extinction phase, the procedures were identical to those of training, except that the drug syringes were removed and extinction occurred in a different nondrug context (context B). Test for reinstatement started after a minimum of 14 daily extinction sessions when the rats met the extinction criterion.
Tests for reinstatement.
For each experiment, the different groups of rats injected systemically or intracranially with SCH 23390 were matched for their number of active lever presses and heroin intake during training and for the number of active lever presses during the extinction phase.
Experiment 1: systemic injections.
In two counterbalanced test sessions (3 h), the rats were injected with vehicle or SCH 23390 before exposure to the training context (A) or the extinction context (B) (tests were performed 48 h apart). SCH 23390 was injected systemically (0, 2.5, or 5.0 μg/kg; three groups; n = 10–11 per group). Each rat was injected in both the training and the extinction contexts with either the vehicle or one dose of SCH 23390.
Experiment 2: accumbens injections.
Nine groups of rats were used. Vehicle or SCH 23390 (0.3 or 0.6 μg/side) was injected into medial shell (three groups; n = 11–16 per group), lateral shell (three groups; n = 8–10 per group), and core (three groups; n = 8–9 per group). Each rat was injected in both the training and the extinction contexts with either the vehicle or one dose of SCH 23390.
Experiment 3: effect of accumbens injections of SCH 23390 on discrete-cue-induced reinstatement.
The experimental procedure consisted of three phases: self-administration training (12 d) in the heroin context (A) in the presence of the discrete tone–light cue, extinction training (10–16 d) in a different nondrug context (B) in the absence of the tone–light cue and heroin, and tests for discrete-cue-induced reinstatement of heroin seeking (2 d) in context B during which lever presses led to contingent tone–light presentations. The physical characteristics of context A and B were counterbalanced. We used this modified discrete-cue-induced reinstatement procedure to avoid a potential influence of the heroin context on the nonextinguished motivational effects of the discrete cues during testing.
Heroin self-administration training and extinction.
Rats were trained to self-administer heroin for 3 h/d for 12 d in context A under an FR1 40 s timeout reinforcement schedule; infusions were accompanied by a discrete tone–light cue for 2.3 s. As in experiments 1 and 2, heroin was infused at a volume of 65 μl over 2.3 s at a dose of 0.1 (first six sessions) and 0.05 (last six sessions) mg/kg per infusion. We used a longer timeout period than the one used in experiments 1 and 2 (2.3 s) because, in previous unpublished studies, we failed to obtain reliable discrete-cue-induced reinstatement of heroin seeking in rats trained under an FR1 2.3 s timeout reinforcement schedule. In contrast, we and others observed reliable discrete-cue-induced reinstatement of heroin seeking in rats trained under an FR1 40 s timeout reinforcement schedule (Fuchs and See, 2002; Bossert et al., 2005a). After training, the rats underwent extinction of lever presses in the absence of the discrete cue in context B.
Tests for reinstatement.
The different groups of rats that were tested for discrete-cue-induced reinstatement were matched for their number of active lever presses and heroin intake during training and for the number of active lever presses during the extinction phase. The rats were tested in context B for discrete-cue-induced reinstatement in test sessions in which lever presses resulted in contingent presentations of the conditioned stimulus (See, 2002), which served as a conditioned reinforcer during testing. In two counterbalanced test sessions (3 h), the rats were injected with vehicle or SCH 23390 before exposure to extinction sessions in the presence (cue condition) or absence (no cue condition) of the discrete tone–light cue. Six groups of rats were used. Vehicle or SCH 23390 (0.6 μg/side) was injected into medial shell (two groups; n = 7 per group), lateral shell (two groups; n = 9 per group), and core (two groups; n = 8–9 per group).
Experiment 4: effect of accumbens injections of SCH 23390 on sucrose self-administration.
We assessed the effect of accumbens SCH 23390 injections on lever presses for 5% sucrose solution to rule out motor deficits of these injections on reinstatement. One week after surgery, food was restricted to maintain 85–90% of the rats' free-feeding weight (∼20 g of food per day). The rats were first trained over 10 d (2 h/d for 2–3 d and 1 h/d for 6–8 d) to lever press for 5% sucrose solution (FR1 reinforcement schedule, 0.2 ml per reward delivery) as described previously (Bossert et al., 2006). When the rats displayed stable responding (<10% variability over 3 d), we examined the effect of vehicle and SCH 23390 (0.3 and 0.6 μg/side) on sucrose self-administration using a counterbalanced within-subjects design. Injections were given every 48 h, and self-administration sessions (with no injections) were given between the test sessions. Vehicle and SCH 23390 were injected bilaterally into the medial and lateral shell and the core (n = 6–7 per group).
Statistical analyses.
Data were analyzed with the statistical program SPSS (general linear model procedure) (SPSS, Chicago, IL), and significant effects (p < 0.05) were followed by Fisher's PLSD post hoc tests. Data from experiments 1–3 were analyzed for total (non-reinforced) active and inactive lever presses. For the statistical analysis of experiments 1–3, the between-subjects factor was SCH 23390 dose and the within-subjects factors were lever (active or inactive) and test context [training (A) or extinction (B)] for experiments 1 and 2 and test condition (cue or no cue) for experiment 3. For the statistical analysis of experiment 4, the within-subjects factors were SCH 23390 dose and lever. The groups of rats tested with different doses of SCH 23390 had similar mean number of lever presses during the training and extinction phases. Thus, nonsignificant group differences during these phases are not reported.
Results
Experiments 1 and 2: effect of systemic and accumbens injections of SCH 23390 on context-induced reinstatement
Training and extinction
Figure 1A shows mean ± SEM number of heroin infusions and presses on the active and inactive levers for all rats that were subsequently tested in experiments 1 and 2 (context experiments). The rats demonstrated reliable heroin self-administration, as indicated by the increase in lever presses when the heroin dose was decreased from 0.1 to 0.05 mg/kg per infusion (p < 0.01). Figure 1B shows the mean ± SEM number of lever presses on the previously active and inactive levers during the first 14 extinction sessions for these rats. As expected, during the extinction phase, response rates decreased over time (p < 0.01).
Figure 1.
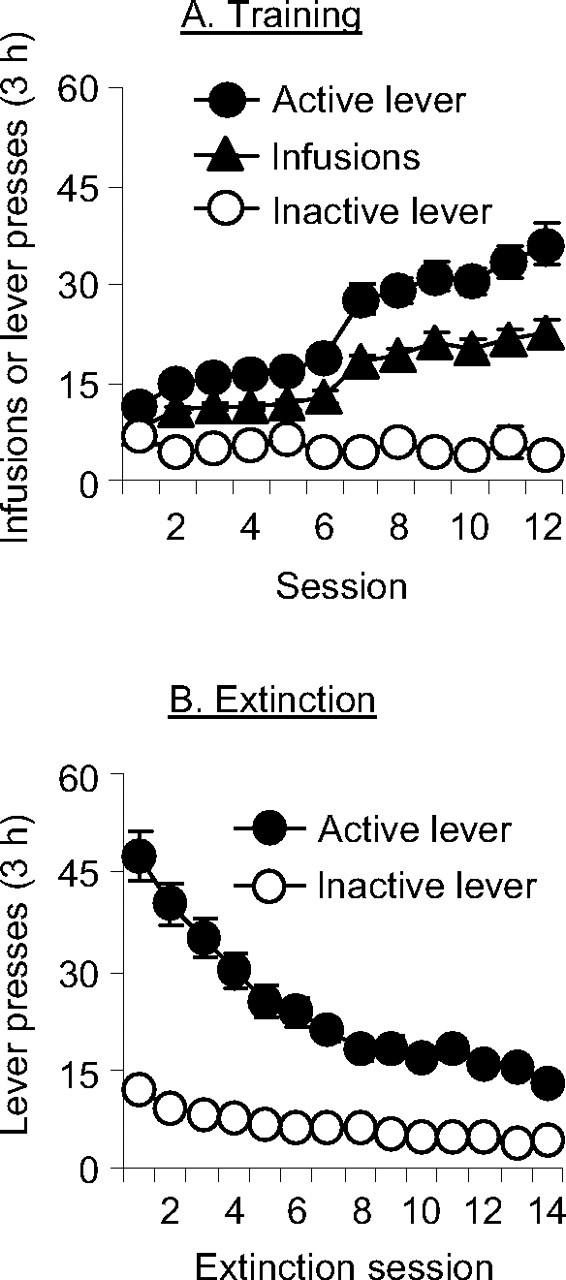
Heroin self-administration training and extinction of the drug-reinforced responding (experiments 1 and 2). A, Training, Mean ± SEM number of infusions and active and inactive lever responses during the 12 d of heroin self-administration training. Rats were trained on a fixed ratio 1 reinforcement schedule with a 2.3 s timeout period; active lever presses reflect infusion plus timeout responses. The unit dose of heroin was 0.1 mg/kg for the first six sessions and 0.05 mg/kg for the last six sessions (n = 125). B, Extinction, Mean number of presses on the previously active lever and on the inactive lever during the first 14 extinction sessions, conducted in the absence of heroin and in a context different from the training context. Data are from all rats in experiments 1 and 2.
Tests for reinstatement
Systemic injections of SCH 23390 attenuated reinstatement of active lever presses (the operational measure of heroin seeking) when rats were exposed to the training (heroin) context after extinction in a different (nondrug) context (Fig. 2). The statistical analysis revealed significant effects of test context (F(1,28) = 75.6; p < 0.01), lever (F(1,28) = 50; p < 0.01), SCH 23390 dose (F(2,28) = 7.5; p < 0.01), SCH 23390 dose × test context (F(2,28) = 6.1; p < 0.01), lever × test context (F(1,28) = 97.6; p < 0.01), SCH 23390 dose × lever (F(2,28) = 4.1; p < 0.05), and SCH 23390 dose × lever × test context (F(2,28) = 6.9; p < 0.01). The higher but not lower SCH 23390 dose also modestly decreased inactive lever presses in the training context and active lever presses in the extinction context, but these effects were not statistically significant (p = 0.29 and p = 0.12, respectively).
Figure 2.
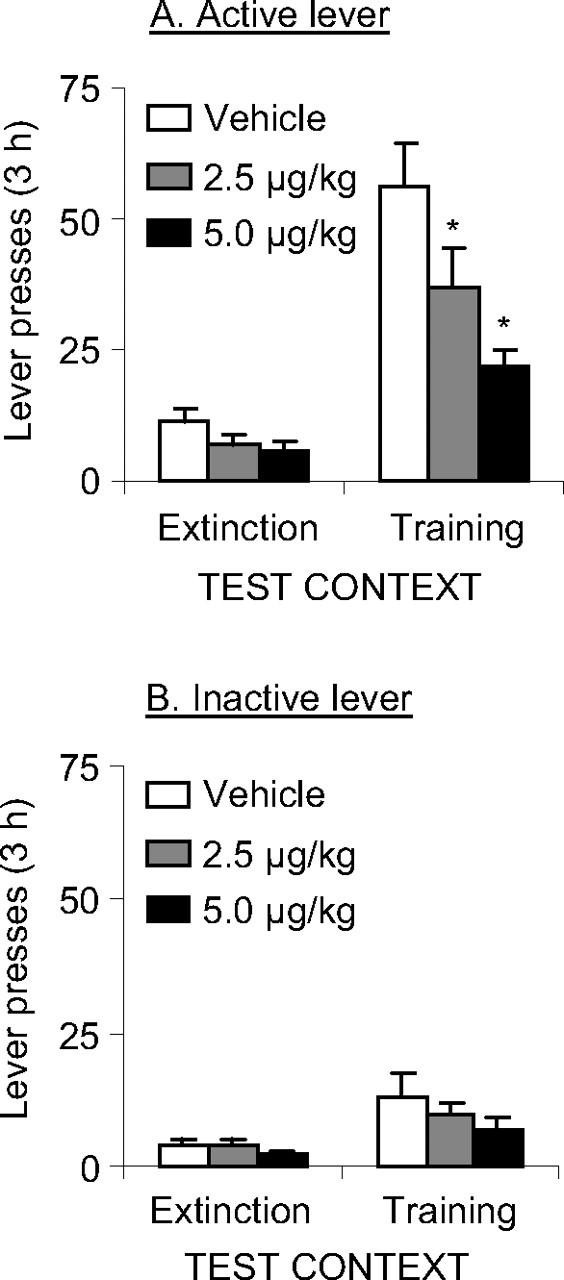
Effects of systemic SCH 23390 injections on context-induced reinstatement of heroin seeking. A, Active lever, Mean ± SEM number of presses on the active lever after injections of vehicle or SCH 23390 before exposure to the training context or the extinction context (n = 10–11 per dose). B, Inactive lever, Mean number of presses on the inactive lever during testing. *p < 0.05, different from the vehicle condition.
SCH 23390 injections into medial and lateral accumbens shell but not core attenuated reinstatement of active lever presses when rats were exposed to the training (heroin) context after extinction in a different (nondrug) context (Fig. 3A). Accumbens SCH 23390 injections had no effect on active lever presses in the extinction context. Figure 3C depicts representative pictures for bilateral cannula placement in medial shell, lateral shell, and core and the approximate injector placements of the injector tips.
Figure 3.
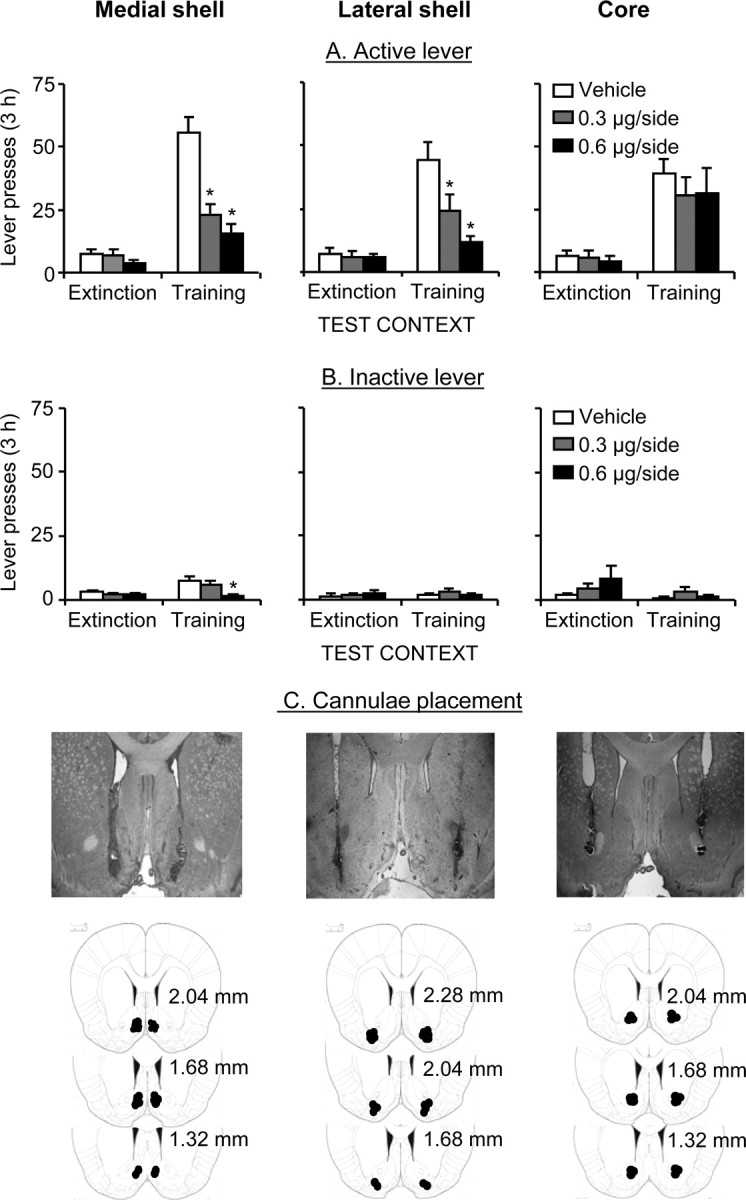
Effects of medial and lateral accumbens shell and accumbens core SCH 23390 injections on context-induced reinstatement of heroin seeking. A, Active lever, Mean ± SEM number of presses on the active lever after bilateral injections of vehicle or SCH 23390 before exposure to the training or the extinction context (n = 8–16 per dose). B, Inactive lever, Mean number of presses on the inactive lever during testing. *p < 0.05, different from the vehicle condition. C, Cannulae placement, Representative pictures of bilateral cannulas and injector placements and approximate placements of the injector tips (Paxinos and Watson, 2005); the numbers on the plates are in millimeters anterior from bregma.
Medial shell.
The statistical analysis (n = 11–16 per dose) revealed significant effects of test context (F(1,39) = 98.7; p < 0.01), lever (F(1,31) = 48.3; p < 0.01), SCH 23390 dose (F(2,39) = 18; p < 0.01), SCH 23390 dose × test context (F(2,39) = 15.5; p < 0.01), lever × test context (F(1,39) = 73.2; p < 0.01), SCH 23390 dose × lever (F(2,39) = 11.5; p < 0.01), and SCH 23390 dose × lever × test context (F(2,39) = 16.6; p < 0.01). SCH 23390 injections also decreased inactive lever presses in the training context (F(2,39) = 4.2; p < 0.05). The interpretation of these inactive lever data are not straightforward. In reinstatement studies, inactive lever pressing is often used as a measure of the effects of drug on nonspecific activity. However, changes in inactive lever presses during tests under extinction conditions can be attributable to the effects of the drug on response generalization (Shalev et al., 2002). Because SCH 23390 at the doses used here had no effect on high-rate lever presses reinforced by a sucrose solution (experiment 4), we suspect that the effects of SCH 23390 on inactive lever presses observed here are likely attributable to its effects on response generalization.
Lateral shell.
The statistical analysis (n = 8–9 per dose) revealed significant effects of test context (F(1,22) = 82.1; p < 0.01), lever (F(1,22) = 43.7; p < 0.01), SCH 23390 dose (F(2,22) = 6.7; p < 0.01), SCH 23390 dose × test context (F(2,22) = 10.5; p < 0.01), lever × test context (F(1,22) = 38.2; p < 0.01), SCH 23390 dose × lever (F(2,22) = 8.4; p < 0.01), and SCH 23390 dose × lever × test context (F(2,22) = 7.5; p < 0.01). SCH 23390 injections had no effect on inactive lever presses.
Core.
The statistical analysis (n = 8–10 per dose) revealed significant effects of test context (F(1,24) = 74.1; p < 0.01), lever (F(1,24) = 30.8; p < 0.01), and lever × test context (F(1,24) = 56.2; p < 0.01). No significant effects were found for SCH 23390 dose, SCH 23390 dose × test context, SCH 23390 dose × lever, or SCH 23390 dose × lever × test context (p values >0.1). SCH 23390 injections had no effect on inactive lever presses.
Experiment 3: effect of accumbens injections of SCH 23390 on discrete-cue-induced reinstatement
Training and extinction
On the last training day, mean ± SEM number of heroin infusions and presses on the active and inactive levers per 3 h for all rats tested in experiment 3 was 18.4 ± 1.6, 118.4 ± 24.6, and 4.5 ± 1.0, respectively. On the first and last extinction session, mean ± SEM number of active lever presses was 33.0 ± 3.9 and 6.2 ± 0.8, respectively. During the extinction phase, lever presses decreased over time (p < 0.01).
Tests for reinstatement
SCH 23390 injections into accumbens core, but not medial or lateral shell, attenuated discrete-cue-induced reinstatement of active lever presses in the extinction (nondrug) context, after extinction of lever presses in the absence of the tone–light cue in this context (Fig. 4A). During testing, active lever presses led to contingent presentations of the tone–light cue but not heroin. Figure 4C depicts representative pictures for bilateral cannula placement in medial shell, lateral shell, and core and the approximate placements of the injector tips.
Figure 4.
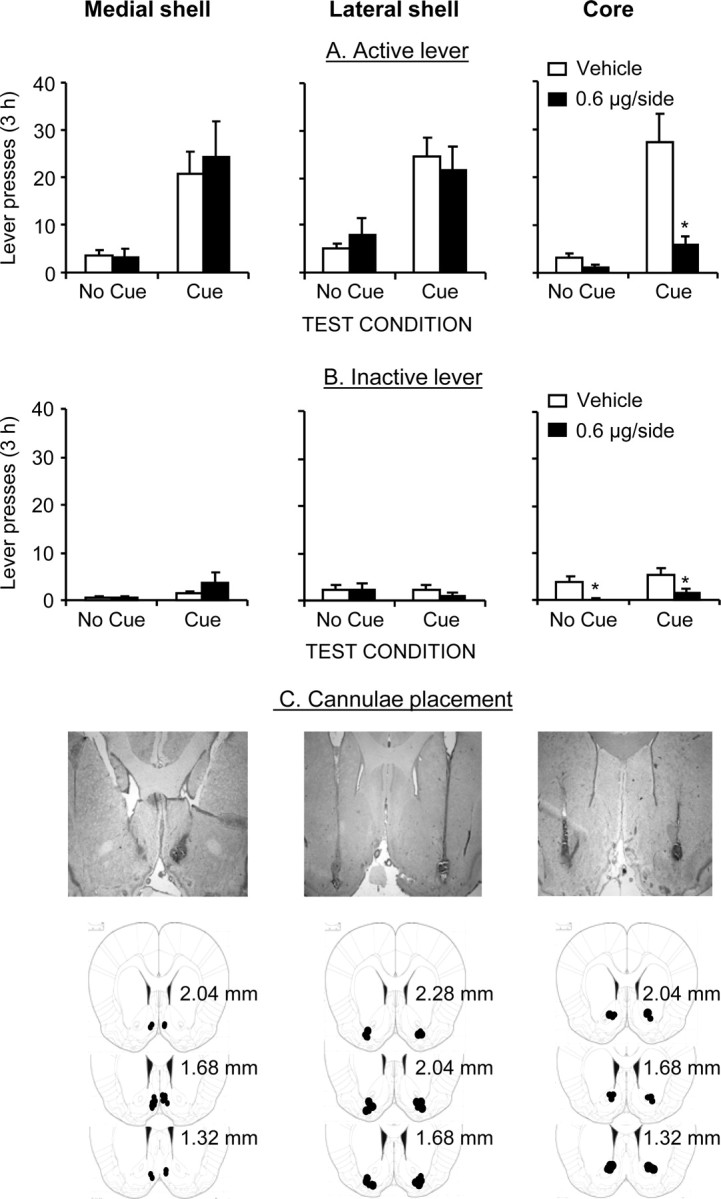
Effects of medial and lateral accumbens shell and accumbens core SCH 23390 injections on discrete-cue-induced reinstatement of heroin seeking. A, Active lever, Mean ± SEM number of presses on the active lever after bilateral injections of vehicle or SCH 23390 before exposure to the cue or no cue condition (n = 7–9 per dose). B, Inactive lever, Mean number of responses on the inactive lever during testing. C, Cannulae placement, Representative pictures of bilateral cannulas and injector placements and approximate placements of the injector tips (Paxinos and Watson, 2005); the numbers on the plates are millimeters anterior from bregma.
Medial shell.
The statistical analysis (n = 7 per condition, 0 and 0.6 μg/side) revealed significant effects of cue condition (F(1,12) = 17.9; p < 0.01), lever (F(1,12) = 30.3; p < 0.01), and lever × cue condition (F(1,12) = 18.3; p < 0.01). No significant effects were found for SCH 23390 dose, SCH 23390 dose × cue condition, SCH 23390 dose × lever, or SCH 23390 dose × lever × cue condition. SCH 23390 injections had no effect on inactive lever presses.
Lateral shell.
The statistical analysis (n = 9 per condition, 0 and 0.6 μg/side) revealed significant effects of cue condition (F(1,16) = 27.3; p < 0.01), lever (F(1,16) = 40.3; p < 0.01), and lever × cue condition (F(1,16) = 30.1; p < 0.01). No significant effects were found for SCH 23390 dose, SCH 23390 dose × cue condition, SCH 23390 dose × lever, or SCH 23390 dose × lever × cue condition. SCH 23390 injections had no effect on inactive lever presses.
Core.
The statistical analysis (n = 8–9 per condition, 0 and 0.6 μg/side) revealed significant effects of SCH 23390 dose (F(1,15) = 15.1; p < 0.01), cue condition (F(1,15) = 26.7; p < 0.01), lever (F(1,15) = 20.5; p < 0.01), SCH 23390 dose × cue condition (F(1,15) = 9.4; p < 0.01), SCH 23390 dose × lever (F(1,15) = 7.6; p < 0.05), lever × cue condition (F(1,15) = 18.5; p < 0.01), and SCH 23390 dose × lever × cue condition (F(1,15) = 9.9; p < 0.01). SCH 23390 injections also decreased inactive lever presses in the cue and no cue condition (p values <0.05).
Experiment 4: effect of accumbens SCH 23390 injections on sucrose self-administration
SCH 23390 injections into medial and lateral accumbens shell and accumbens core had no effect on sucrose self-administration (p values >0.1) (Fig. 5).
Figure 5.
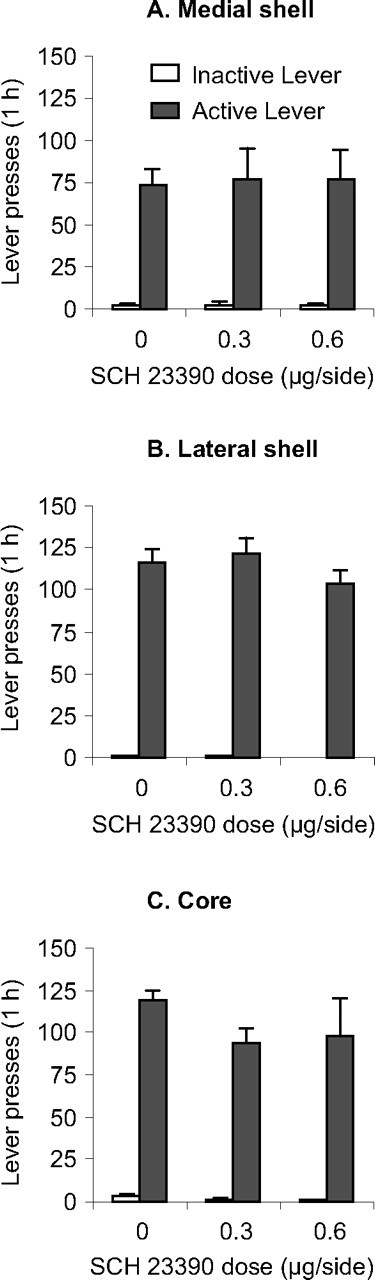
Effect of accumbens SCH 23390 injections on sucrose self-administration. Mean ± SEM number of responses on the active lever after bilateral injections of vehicle or SCH 23390 (n = 6–7 per accumbens site). No significant effects were observed.
Discussion
We found that blockade of D1-family receptors in medial and lateral accumbens shell but not core decreased context-induced reinstatement of heroin seeking, whereas blockade of these receptors in accumbens core but not medial and lateral shell decreased discrete-cue-induced reinstatement. These results demonstrate dissociable roles of accumbens core and shell in context- versus discrete-cue-induced reinstatement of heroin seeking and provide the first demonstration for a role of lateral accumbens shell in conditioned drug effects.
Role of accumbens shell and core in cue-induced drug seeking
Accumbens core and shell are heterogeneous structures with distinct immunohistochemical characteristics and afferent and efferent connections (Voorn et al., 1989; Zahm and Brog, 1992). These neuroanatomical findings led to many studies on core and shell roles in motivated behavior (Cardinal et al., 2002; Kelley, 2004) and conditioned and unconditioned rewarding effects of drugs (Everitt and Wolf, 2002; Di Chiara et al., 2004; Ikemoto and Wise, 2004). Our results demonstrating dissociable roles of accumbens core and shell in context- versus discrete-cue-induced reinstatement of heroin seeking are in agreement with the notion that core and shell mediate different aspects of drug-motivated behaviors (Everitt and Robbins, 2005).
Our results on the role of shell but not core in context-induced reinstatement of heroin seeking are in agreement with our previous findings that medial shell injections of the mGluR2/3 agonist LY379268 decreased context-induced reinstatement of heroin seeking at doses 10 times lower than those required to attenuate this reinstatement after core injections (Bossert et al., 2006). Our present and previous results are also consistent with those of Hamlin et al. (2006, 2007) demonstrating that context-induced reinstatement of alcohol and sucrose seeking is associated with increased Fos expression in medial shell, an effect reversed by systemic SCH 23390 injections. The present results also agree with those of Ghitza et al. (2003) who reported that exposure to discriminative cues that predicted cocaine availability increases neuronal activity in shell but not core.
The present results on the role of D1-family receptors in accumbens core but not shell in discrete-cue-induced reinstatement of heroin seeking are consistent with data from several studies using cocaine-trained rats. Fuchs et al. (2004) reported that reversible inactivation (muscimol plus baclofen) of core but not shell decrease discrete-cue-induced reinstatement of cocaine seeking. Everitt and colleagues reported that permanent lesions or antagonism of AMPA receptors in core but not shell decrease discrete-cue-induced cocaine seeking, as assessed in a second-order reinforcement schedule (Di Ciano et al., 2001; Ito et al., 2004). Using a reversible inactivation procedure, these investigators also demonstrated a role of core but not shell in discrete-cue-induced cocaine seeking, as assessed in an acquisition of a new response procedure (Di Ciano et al., 2007).
Together, our results and those reviewed above suggest that activation of accumbens core neurons mediates discrete-cue-induced drug seeking, whereas activation of accumbens shell neurons mediates context-induced drug seeking. However, one issue to consider before accepting this idea is that, whereas in context-induced reinstatement studies cues are presented independent of the rats' behavior, in studies using discrete-cue-induced reinstatement (See, 2002) or second-order schedule (Goldberg, 1976) procedures, cue presentations are made contingent on lever presses, i.e., the discrete cue functions as a conditioned reinforcer during testing. Thus, an alternative interpretation of the present and previous results is that core–shell differences in motivational effects of drug cues reflect anatomical differences in neuronal responses of these subregions to the contingency of cue presentations. Unfortunately, empirical testing of this hypothesis is not straightforward because, whereas noncontingent exposure to contextual (Crombag and Shaham, 2002) or discriminative (Weiss et al., 2000) cues reliably reinstate drug seeking after extinction, noncontingent presentations of discrete cues do not (Grimm et al., 2000).
The present data on the role of medial shell in context-induced reinstatement of heroin seeking are in some agreement with results demonstrating a role of this accumbens subregion in conditioned effects of opiate drugs. Bassareo et al. (2007) reported that conditioned cues paired previously with morphine injections increase dopamine release in shell but not core. Harris and Aston-Jones (2003) reported that Fos (a neuronal activity marker) induction after exposure to morphine-paired contexts is more pronounced in shell than in core. However, Sellings and Clarke (2003) reported that 6-OHDA shell lesions have no effect on the acquisition or expression of morphine conditioned place preference (CPP), and Fenu et al. (2006) reported that, although medial shell SCH 39166 (a D1-family receptor antagonist) injections prevent acquisition of morphine CPP, these injections have no effect on CPP expression. Integration of findings from studies on conditioned opiate effects in which rats are given noncontingent morphine injections in a distinct context with our findings on context-induced reinstatement of operant responding, however, should be done with caution. It is likely that different learning and motivational processes mediate context-induced reinstatement of operant responding after contingent heroin self-administration versus opiate CPP.
Finally, there are anatomical differences between medial and lateral accumbens shell (Meredith, 1999; Voorn et al., 2004), and these subregions play different roles in mediating drug and food reward (Kelley, 2004; Ikemoto, 2007). Also, Jansson et al. (1999) reported that D1 receptor expression is higher in medial than in lateral shell. Our data, however, suggest that, for context-induced reinstatement of heroin seeking, dopamine receptor signaling in lateral and medial shell appear equally important.
Methodological considerations
It is unlikely that the effects of systemic or accumbens SCH 23390 injections on reinstatement are attributable to motor deficits. The anatomical specificity of SCH 23390 effects on context- versus discrete-cue-induced reinstatement of lever presses indicates that motor deficits cannot account for our data. Also, although systemic or accumbens SCH 23390 injections had some effect on inactive lever presses during testing (see Results), systemic (5–10 μg/kg) (Crombag et al., 2002) or accumbens SCH 23390 injections had no effect on high-rate sucrose self-administration. However, data from drug-naive sucrose-trained rats should be interpreted with caution in the absence of data indicating that chronic heroin exposure does not increase sensitivity to SCH 23390 cataleptic effects.
In vitro studies indicate that SCH 23390 is an agonist of 5-HT2C (previously 5-HT1C) receptors (Hoyer et al., 1989; Briggs et al., 1991), and Fletcher et al. (2007) reported that the 5-HT2C agonist Ro60-0175 [(S)-2-(6-chloro-5-fluoroindol-1-yl)-1-methylethylamine) fumarate] attenuates context-induced reinstatement of cocaine seeking. However, it is unlikely that the effect of SCH 23390 on context-induced reinstatement is mediated by 5-HT2C receptors. Bischoff et al. (1986) reported that high systemic doses (3–10 mg/kg) of SCH 23390 bind to 5-HT receptors in frontal cortex but not in striatum or hippocampus.
An issue to consider in the interpretation of our data is that we used different timeout periods in experiments 1 and 2 (2.3 s, context) and experiment 3 (40 s, discrete cue) that led to higher response rates during training in experiment 3. It is unlikely, however, that differences in response rates during training confound the interpretation of our data, because there is little evidence from many reinstatement studies that differences in response rates during training cause qualitative changes in the effects of pharmacological manipulations on reinstatement after extinction (Shalev et al., 2002).
The effect of accumbens shell SCH 23390 injections on context-induced reinstatement may be attributable to ventricular diffusion. This issue is particularly relevant to our context-induced reinstatement medial shell results, because our original stereotaxic surgery cannulas were implanted at a 6° angle, which passed through the lateral ventricles. Results from an angiotensin drinking test (Johnson and Epstein, 1975) suggest ventricular diffusion of SCH 23390 (see Materials and Methods). However, as mentioned in Materials and Methods, we tested additional rats with 25° angle cannulas that do not penetrate the ventricles and found robust attenuation of context-induced reinstatement by SCH 23390 in these rats. These results and those on the effect of lateral shell SCH 23390 injections (which are farther away from the ventricles) on context-induced reinstatement indicate that ventricular diffusion of SCH 23390 cannot explain its effect on this reinstatement after accumbens shell injections.
The effects of lateral or medial shell SCH 23390 injections may be attributable to diffusion to the other shell subregion. This is an unlikely possibility because, for context-induced reinstatement, the distance between the ineffective core injection sites to lateral or medial shell injection sites was shorter than the distance between lateral and medial shell injection sites. The effects on context-induced reinstatement of medial and lateral shell SCH 23390 injections, however, may be in part attributable to diffusion into medial or lateral olfactory tubercle. This is an important issue in light of recent studies on similarities in behavioral effects of psychostimulant drug injections in medial accumbens shell and medial tubercle versus lateral accumbens shell and lateral tubercle (Ikemoto, 2003; Ikemoto et al., 2005). These behavioral and anatomical data on similarities between afferent and efferent connections of medial tubercle and medial accumbens shell versus lateral tubercle and lateral accumbens shell led Ikemoto (2007) to propose that medial and lateral tubercles are anatomical extensions of the medial and lateral accumbens shell. Within this new framework, our data are interpreted to suggest that both “ventromedial striatum” (medial accumbens shell–medial tubercle) and “ventrolateral striatum” (lateral accumbens shell–lateral tubercle) dopamine receptors contribute to context-induced reinstatement of heroin seeking.
Conclusions
Using a reinstatement model, an animal model of relapse to drugs (Epstein et al., 2006), we found that lateral and medial shell but not core SCH 23390 injections attenuate context- but not discrete-cue-induced reinstatement of heroin seeking. In contrast, core but not shell SCH 23390 injections attenuate discrete-cue- but not context-induced reinstatement. These results demonstrate dissociable roles of accumbens core and shell in context- versus discrete-cue-induced reinstatement of heroin seeking. In addition, the present results provide the first demonstration for lateral accumbens shell role in conditioned drug effects; in all previous lesion and microinjections studies on conditioned drug effects, manipulations were limited to medial shell and core (Wise, 2004; Everitt and Robbins, 2005). Our results raise two questions for future research: the generality of the findings to reinstatement of cocaine seeking and whether core–shell dissociation for context- versus cue-induced reinstatement can be demonstrated with D2-family receptor antagonists. Finally, although current neurobiological addiction theories emphasize accumbens core role in drug relapse (Everitt and Robbins, 2005; Kalivas et al., 2005), our results suggest that accumbens shell activity also contributes significantly to this relapse.
Footnotes
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse. We thank Dr. Satoshi Ikemoto for lateral accumbens shell coordinates and placement confirmation, Jamie Uejima, Sam Golden, and John McClure for technical help, and Drs. Gloria Meredith and Geoffrey Schoenbaum for helpful comments. This manuscript is dedicated to the late Dr. Ann Kelley whose seminal work on the role of nucleus accumbens core and shell in motivated behavior inspired us to conduct the experiments reported in this manuscript.
References
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology. 2007;191:689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Heinrich M, Sonntag JM, Krauss J. The D-1 dopamine receptor antagonist SCH 23390 also interacts potently with brain serotonin (5-HT2) receptors. Eur J Pharmacol. 1986;129:367–370. doi: 10.1016/0014-2999(86)90449-8. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. NeuroReport. 2005a;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005b;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:445–466. [Google Scholar]
- Briggs CA, Pollock NJ, Frail DE, Paxson CL, Rakowski RF, Kang CH, Kebabian JW. Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SKF 38393. Br J Pharmacol. 1991;104:1038–1044. doi: 10.1111/j.1476-5381.1991.tb12546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burattini C, Gill TM, Aicardi G, Janak PH. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience. 2006;139:877–887. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Crombag H, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1007–1016. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301522. in press. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G. Morphine-conditioned single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology. 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) receptor agonist Ro60–0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301509. in press. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology. 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacol Rev. 1976;27:325–340. [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Waeber C, Schoeffter P, Palacios JM, Dravid A. 5-HT1C receptor-mediated stimulation of inositol phosphate production in pig choroid plexus. A pharmacological characterization. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:252–258. doi: 10.1007/BF00173573. [DOI] [PubMed] [Google Scholar]
- Hurd YL, McGregor A, Ponten M. In vivo amygdala dopamine levels modulate cocaine self-administration behaviour in the rat: D1 dopamine receptor involvement. Eur J Neurosci. 1997;9:2541–2548. doi: 10.1111/j.1460-9568.1997.tb01683.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.05.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of d-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jansson A, Goldstein M, Tinner B, Zoli M, Meador-Woodruff JH, Lew JY, Levey AI, Watson S, Agnati LF, Fuxe K. On the distribution patterns of D1, D2, tyrosine hydroxylase and dopamine transporter immunoreactivities in the ventral striatum of the rat. Neuroscience. 1999;89:473–489. doi: 10.1016/s0306-4522(98)00317-0. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Epstein AN. The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res. 1975;86:399–418. doi: 10.1016/0006-8993(75)90891-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann NY Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Agolia R, Arts MP, Groenewegen HJ, Zahm DS. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–162. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Mclellan TA, Ehrman R. Classical conditioning in drug dependent humans. Ann NY Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Amsterdam: Elsevier Academic; 2005. The rat brain in stereotaxic coordinates, Ed 5. [Google Scholar]
- Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci. 2001;21:5841–5846. doi: 10.1523/JNEUROSCI.21-15-05841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking under opioid maintenance: the effects of opioid withdrawal, heroin priming and stress. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smit DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]


