Abstract
We examine Medicare costs and survival gains for Acute Myocardial Infarction (AMI) during 1986–2002. Like Cutler and McClellan, we find overall gains in post-AMI survival more than justified the increases in costs during this period. Since 1996, however, survival gains have stagnated, while expenditures have continued to increase. We also consider changes in expenditures and outcomes at the regional level. Regions experiencing the largest expenditure gains were not those realizing the greatest improvements in survival. Factors yielding the greatest benefits to health (aspirin, beta blockers, and reperfusion) were not the factors that drove up costs (multiple physicians), and conversely.
In a series of important papers, David Cutler, Mark McClellan, and their coauthors have argued persuasively that the benefits from many technological innovations more than justify the rising costs of health care. 1 While they recognized the presence of waste in the U.S. health care system, the policy message is clear: don’t kill the golden goose of future medical advances in pursuit of short-sighted cost savings.
Cutler and McClellan summarized research for five diseases, but the most striking evidence came from the rapid decline in mortality following heart attacks or acute myocardial infarction (AMI). Briefly, they found that between 1984 and 1998, the costs of treating heart attacks rose by ten thousand dollars in real terms, but life expectancy increased by about one year. In short, technological innovations in the treatment of cardiac disease provided terrific value for the dollar; in this case, the rising costs were “worth it.”2
In contrast to the studies by these authors and others studying the benefits of technological advances in health care, 3 there is another set of studies that call into question the assumption that spending more on medical care leads inexorably to improved outcomes. These have focused on differences in costs and outcomes across regions. 4 Fisher and his colleagues, for example, have found that Medicare patients in regions with higher levels of health care expenditures do not experience better health outcomes, nor do they gain better access to care or report greater satisfaction. More recently, a study using state-level data demonstrated a negative association between quality of care and health care expenditures. 5
This paper strives to reconcile these two seemingly contradictory sets of findings by returning to the original analysis on AMI mortality and expenditures by Cutler, McClellan, and their coauthors, but with an expanded dataset that stretches from 1986–2003. We first ask whether the survival gains observed in the earlier data have continued into the current century: have recent increases in health care costs still been worth it? We then ask whether regions experiencing the most rapid improvements in health outcomes were also the regions experiencing the most rapid increase in costs. Were there specific regional characteristics, such as early adoption of low-cost highly effective treatments such as β Blockers, aspirin, and reperfusion, or a reliance on care by multiple medical specialists, that may have been associated with unusually rapid survival gains or with unusually low increases in expenditure? In the final section of the paper, we suggest a simple fra mework that reconciles the two prevailing views of health care technology.
Data and Methods
The Medicare Part A hospital claims data from 1986–2003 were merged with the Medicare Denominator File through 2003 to create a longitudinal cohort of fee-for-service enrollees age 65 or over coded with a new acute myocardial infarction. 6 During 1986–1991 the sample of Part A data was 20 percent of the fee-for-service Medicare population, rising to 100 percent since 1992. Patients with a code of “old MI,” or those identified from the panel data as having had an AMI previously, were excluded from the sample. 7 Overall there were 2,872,050 valid AMI events. For pedagogical reasons, we find it helpful to present our results in terms of survival, or the percentage of AMI patients surviving to one year following their AMI. Expenditure data are available only through the end of 2003, so one-year survival and expenditure data are analyzed from 1986–2002.
The primary analysis follows Cutler and McClellan by using just the Medicare Part A hospital expenditures, correcting for inflation using the US implicit price deflator 8 and expressing all results in 2003 dollars. Both expenditures and survival rates are determined for the same one-year horizon. We also provide secondary analysis using a smaller sample of Part B expenditure data: a 5% sample in 1993–1997 and a 20% sample from 1998–2002.
To adjust for both secular and cross-sectional differences in health status, we adjust survival rates and expenditures for a variety of comorbidities (diabetes, diabetes with complications, pulmonary disease, liver disease, liver disease with complications, dementia, non-metastic cancer, metastatic cancer). Also included were age-sex-race effects consisting of 5 age categories (65–69, 70–74, 75–79, 80–84, and 85+) interacted with sex and with two race variables (black and nonblack), and the type of MI (inferior, anterior, subendocardial, and other).
The regression analysis explains survival and expenditures as a linear function of demographic variables, comorbidities, type of AMI, and year categorical variables. 9 All estimated survival and expenditure measures are expressed in terms of the representative patient with average characteristics during the entire period of analysis using the ADJUST command in STATA 9. Thus the regression adjusts for changes over time in the severity of the disease, demographic changes in the Medicare population, and general increases in the price level.
The regional unit of analysis is the Hospital Referral Region (HRR), which was constructed in the Dartmouth Atlas of Health Care to reflect the actual hospital migration patterns of Medicare patients for tertiary care. 10 There are 306 HRRs in the United States, and each must include at least one hospital that performs cardiac surgery and neurosurgery. Each zip code in the United States is assigned to an HRR depending on what hospital the majority (or in some cases, the plurality) of Medicare enrollees seek their hospital care, so the HRR may cross county or state boundaries. Individuals were assigned to HRRs depending on their zip code of residence, and not whether they were actually admitted to hospitals in those HRRs.
Region-specific measures of annual survival rates and cost measures were constructed from the estimated linear regressions mentioned previously that control for comorbidities and demographics. These region-year-specific measures are interpreted as the risk-adjusted survival rate and expenditures for the representative Medicare AMI patient in that region and year. Regions will differ both with regard to their initial adjusted survival and expenditures, and with respect to changes over time in these variables, but our approach will, as far as possible, ensure that the results reflect regional practice patterns rather than regional differences in patient characteristics. These adjusted measures are used both in the cross-sectional analysis (using just 2002 data) and the longitudinal analysis that examines changes over time 1986–2002.
There are a variety of approaches to treating patients with AMI, and as we show below, regions differed dramatically in their adoption of treatment strategies such as aspirin, β blocker use, and reperfusion, as well as their reliance upon multiple physicians per patient. We hypothesize that regional differences in the diffusion of new treatment strategies will be associated with survival gains and expenditures increases during 1986–2002. Note that we conduct our hypothesis tests at the regional level rather than at the individual patient level. Unobservable aspects of specific patients will lead to unmeasured confounding factors and resulting biases. Differences across region, on the other hand, are small with regard to the average severity of heart attacks, but large with regard to treatment strategy.
In the regression analysis, we focus on two region-level dimensions of care. The first is an index of low-cost highly effective treatments for AMI: aspirin at discharge, β Blockers at discharge, and reperfusion within 12 hours of admission (whether surgical reperfusion or thrombolysis). Aspirin reduces platelet aggregation and is known to reduce the risk of mortality following AMI. 11 Beta blockers are an inexpensive drug that by blocking the beta-adrenergic receptors reduces the demands upon the heart, and have been known since the mid-1980s to be effective in reducing post-AMI mortality by 25 percent or more. 12 Compliance in the use of β Blockers has lagged among many regions, even as late as 2000/2001. 13 Reperfusion encompasses either thrombolytics, “clot-busting” drugs designed to improve blood flow in the blocked arteries, or percutaneous transmural coronary angioplasty (PTCA) within 12 hours of the AMI, again with well-established reductions in the risk of death. 14
Measures are the percentage of patients in each region deemed ideal for treatment who actually did receive treatment, and are based on chart reviews from the Cooperative Cardiovascular Project (CCP) survey, and reported in the Dartmouth Atlas of Cardiovascular Care. 15 These data are available only in 1994/95, but these were years marked by a remarkable divergence in the adoption of these treatments. For example, β Blocker prescription at discharge among ideal heart attack patients was 20 percent in San Antonio, TX, 42 percent in Orange County, CA, and 82 percent in Rochester, NY. Thus 1994/95 data allows us to identify early and laggard adopters. 16 Regional quality is determined by the number of quality measures for which that region is above the national median. The quality measure ranges from 0 (below median for all 3 measures) to 3 (above median for all three measures).
Our second dimension of care is the average number of different physicians treating the patient within one year following the AMI, averaged across all patients in the Hospital Referral Region in 1994/95. This measures both the degree of reliance on specialists, as well as a marker for the continuity of care. There are potential gains from the specialization of medical knowledge, but there are also “network” costs associated with a larger number of interactions necessary among physicians coordinating care. 17 For example, when there are 4 physicians treating a given patient, there are 4×3/2 = 6 possible interactions (whether communication among physicians or potential for drug regimen interactions). With 8 physicians the number of interactions rises to 28, or a 367% increase in the number of potential interactions. Their potential adverse effects are illustrated in a recent Newsweek column written by the wife of a severely ill man:
… the cardiologist told me that Doug was doing reasonably well, and I naively took solace in this mild pronouncement. That is, until a lung specialist zipped into the room, put his stethoscope to Doug’s chest and said ‘He’s not getting any better. He’s worse. He may die. Any questions?’ I was too stunned to be coherent.
Later a nephrologist informed me that Doug’s kidneys were failing and he needed dialysis. I told this doctor what the prior two specialists had said, hoping he could reconcile their conflicting reports. Instead, he plied me with questions about their findings that I could not answer. 18
In other words, the apparent lack of communication among the specialists could attenuate (or even offset) the advantages of specialization. 19
We hypothesize that regions where quality measures were adopted early would experience the greatest improvement in survival with small influences on Medicare expenditures. By contrast, we hypothesize that a larger number of separate physicians should have uncertain effects on survival (depending on whether the network effects overwhelm the gains from specialization) but are likely to be associated with more rapid increases in expenditures during the period. We are not estimating a traditional economic “production function” in part because we do not have sufficient data on inputs in each year 1986–2002. Instead, we test whether characteristics of regions in 1994/95 are predictive of productivity growth; that is, whether survival gains are high relative to expenditure growth over the period 1986–2002.
To quantify the importance of these two region-level measures (quality treatment and number of different physicians), a least-squares regression is estimated at the HRR-level. The dependent variable is the risk-adjusted change, 1986–2002, in one-year survival, one-year expenditures, or one-year log (or proportional) expenditures. The index of quality is entered flexibly with separate categorical variables for the quality index from 0 to 3, and for quartiles (or 25th percentile groupings) of the average number of different physicians per patient, with all estimates reported using the ADJUST command in STATA 9.
The Cross Sectional Evidence
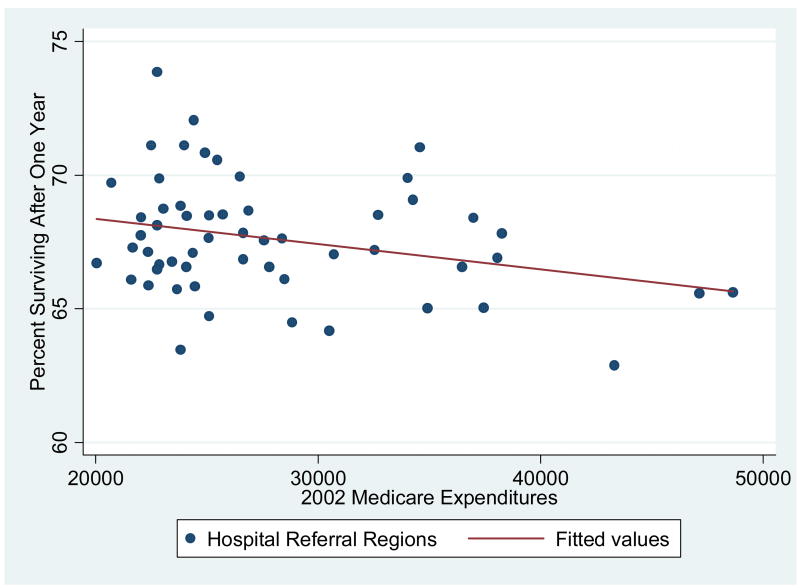
Exhibit 1 displays the association between 2002 Medicare Expenditures and adjusted 2002 one-year survival for the 56 larger HRRs with at least 250 cases of AMI in the base year of 1986, along with the predicted regression line. There are very wide differences across regions: in Knoxville, TN adjusted one-year survival was 69.7 per 100 AMI patients, with adjusted one-year expenditures equal to $20,720. By contrast, risk-adjusted survival was 65.6 per 100 AMI patients in New York City, with expenditures of $47,133. In the entire sample of 306 HRRs, the (weighted) correlation coefficient between survival and expenditures is −0.33 (p < .001).20 As in previous studies, there is no evidence that higher levels of expenditures are associated with better outcomes.
Exhibit 1. The Association Between Adjusted One-Year Survival Post-AMI and One-Year Expenditures by Hospital Referral Region: 2002.
Legend: Each dot represents a hospital referral region (HRR) with at least 250 individuals experiencing a heart attack in 1986. (N = 56) The sample is the elderly (65+) fee-for-service Medicare population, and both survival rates and expenditures are adjusted for age, sex, rate, comorbidities, and the severity of the heart attack.
Focusing on just Part A (inpatient) expenditures could be misleading because some regions could provide a greater fraction of care through Part B (physician) expenditures. However, the correlation between total (Parts A and B) expenditures and survival is similar (ρ = −.36, p < .001), largely because Part A and Part B expenditures are themselves so highly correlated (ρ = .77, p < .001). In sum, these results are at least consistent with the interpretation that U.S. health care is on the “flat of the curve” where spending more does not yield additional health benefits; indeed it seems to imply (from the least-squares regression line) that more spending yields worse health outcomes.
The Time-Series Evidence on Survival
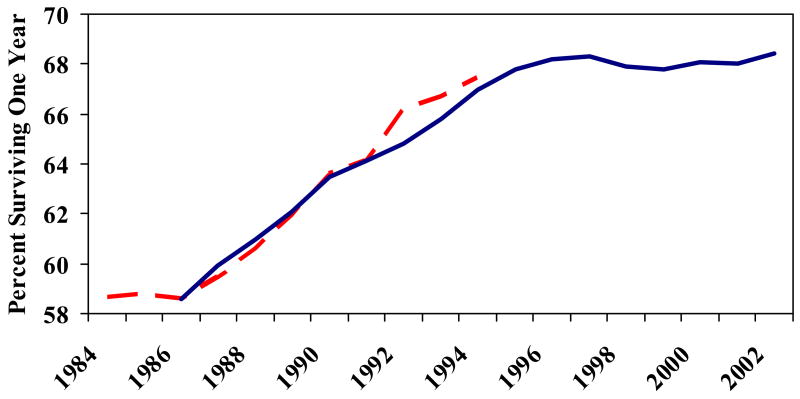
Exhibit 2 displays two measures of year-specific survival rates. The first is for the period 1984–94 from the original Cutler and McClellan study, converted to survival rates. 21 The second series is based on our own data analysis from 1986 through 2002, using data on survival through the end of 2003. There is a close correspondence between the two series during the overlapping years. What is perhaps most surprising is the flattening of mortality following 1996, a result also found in Medicare claims data during 1996–99 by Ash et al. 22 This may appear puzzling, given both continued diffusion of treatments such as beta blockers, and continued technological developments such as stents, cylindrical wire mesh inserted in the arteries to maintain blood flow following angioplasty. However, by the mid-1990s the diffusion of aspirin – perhaps the largest contributor to improvements in survival during this period – had begun to slow, while a meta-analysis of stents during this period did not show significant mortality effects. 23
Exhibit 2. Adjusted One-Year Survival For Elderly Medicare Enrollees with an AMI: 1986–2002.
Legend: The vertical axis measures the percentage who survive the index acute myocardial infarction (AMI). This is equal to 100 minus the percentage one-year mortality. The left dashed line (1984–94) is from D. M. Cutler and M. McClellan, “Is Technological Change in Medicine Worth It?” The right solid line (1986–2002) is from the authors’ calculations.
The Time-Series Evidence on Expenditures
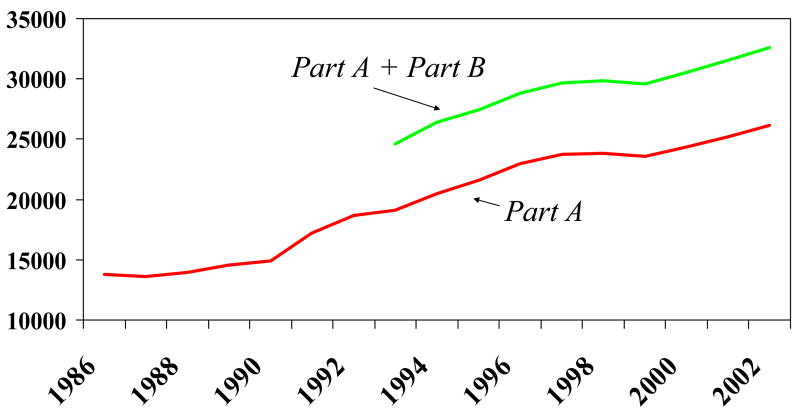
Exhibit 3 displays the corresponding trend in average Part A Medicare annual expenditures, and total expenditures, during the period 1986–2002, and the corresponding combined Part A and Part B expenditures from 1993–2002. While the rise in Medicare expenditures slowed during the late 1990s, largely because of the 1997 Balanced Budget Act, they have since resumed an upward trend.
Exhibit 3. Adjusted One-Year Medicare Expenditures For Elderly Medicare Enrollees with an AMI: 1986–2002.
Legend: The vertical axis measured risk-adjusted real Medicare expenditures following the index acute myocardial infarction (AMI) for each year 1986–2002. All expenditures are adjusted for inflation using the GDP Deflator. The higher line includes Part B expenditures, from 1993–2002.
As a simple benchmark, we note that Part A expenditures increased by $12,399 during 1986–2002 (in $2003). At the same time, expected one-year survival has risen by 9.8 per 100 AMI patients. Using the adjustment in the Cutler et. al. 1998 study to transform one-year mortality to expected life years, the average cost-effectiveness ratio is $23,723 per life-year which in 2003 dollars means that the health care costs over the entire period were “worth it” in the sense of meeting standard cost-effectiveness criterion. Since 1996, however, the cost effectiveness ratio for Part A expenditures was just under $300,000, a number well above traditional cost-effectiveness hurdle and thus no longer “worth it.”
Still, we are left with a puzzle; why does the cross-sectional data tell such a different story from the time-series patterns from 1986 to 2002? A better understanding of this puzzle can be gained by considering region-specific changes over time in survival and expenditures, to which we turn next.
Regional Changes in Survival and Expenditures
One simple hypothesis is to ask whether regions that experienced the greatest cost increases during 1986–2002 also experienced the greatest gain in survival during the same period. We consider this question using both graphical and statistical approaches. Again, in the graph we include only regions with at least 250 AMI patients in 1986, while the statistical analysis uses all data at the HRR level, weighted by the number of AMI patients in 1986.
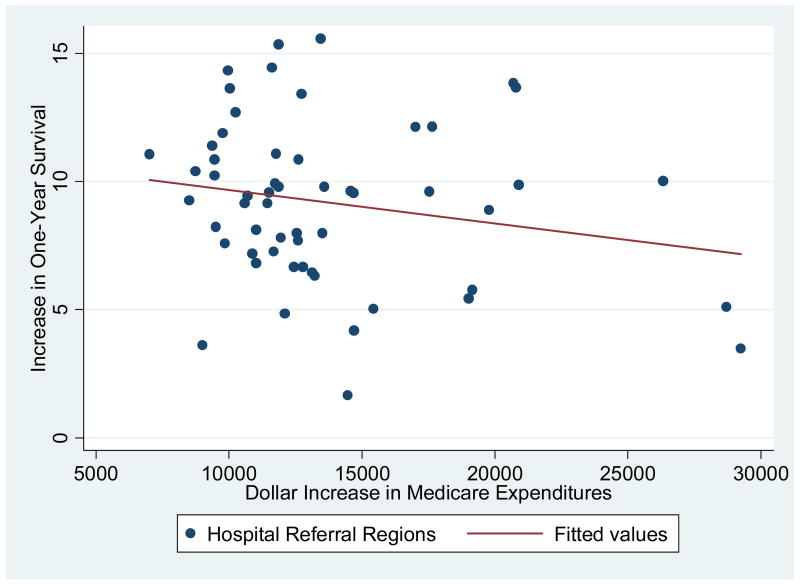
Exhibit 4 shows the average real dollar increase in one-year expenditures per patient between 1986 and 2002 on the horizontal axis and the average increase in survival (per 100 patients) on the vertical axis. Every region benefited from the technological revolution in the treatment of heart attack patients. But some regions experienced much greater benefits than others. As well, every region experienced an inflation-adjusted increase in expenditures, again with some regions experiencing greater increases than others.
Exhibit 4. The Change in Survival and the Change in Medicare Expenditures by Hospital Referral Regions: 1986–2002.
Legend: Each dot represents a hospital referral region (HRR) with at least 250 individuals experiencing a heart attack in 1986. (N = 56) The sample is the elderly (65+) fee-for-service Medicare population, and both survival rates and expenditures are adjusted for age, sex, rate, comorbidities, and the severity of the heart attack.
If simply spending more on health care improved survival, we would expect a positive association between survival and expenditures, with regions lining up from the Southwest to the Northeast of the graph. However, if anything the association is reversed; regions experiencing the greatest increase in expenditures during 1986–2002 tended to lag behind in survival gains.
The graphical result is born out in statistical correlations using the entire data set. The correlation coefficient between growth in survival and in expenditures is −0.21 (p < .001). These dollar increases in expenditure reflect both changes in reimbursement rates across regions, and in the quantity of care provided. Focusing just on the quantity of care, we created an index of the billing weights from the inpatient diagnostic-related groups (DRG). This index is not affected by either regional differences in reimbursement rates or changes in reimbursement rates. Again, the correlation coefficient is −0.14 (p = .02). Finally, the correlation between the proportional growth rate in expenditures and in survival is −0.04 (p = .49). At best, there is no association between expenditure growth and survival gains.
Factors Associated With Changes in Regional Mortality and Expenditures
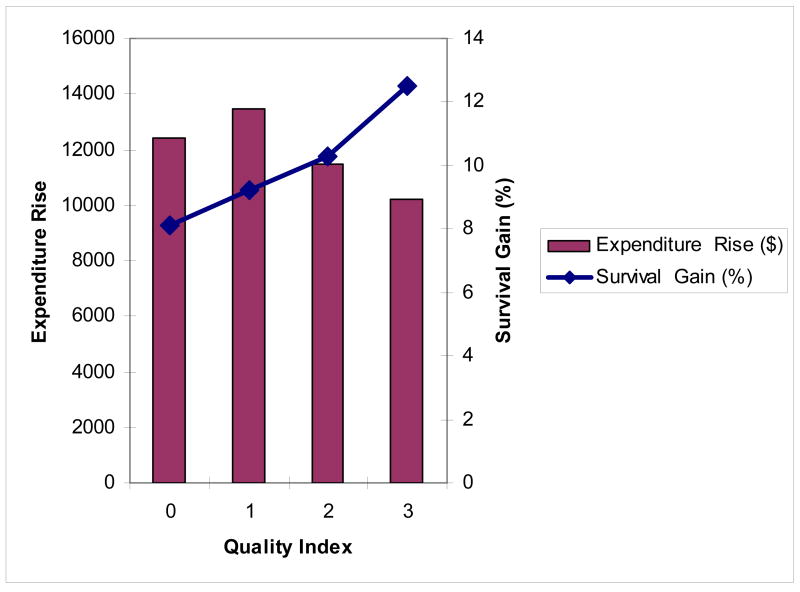
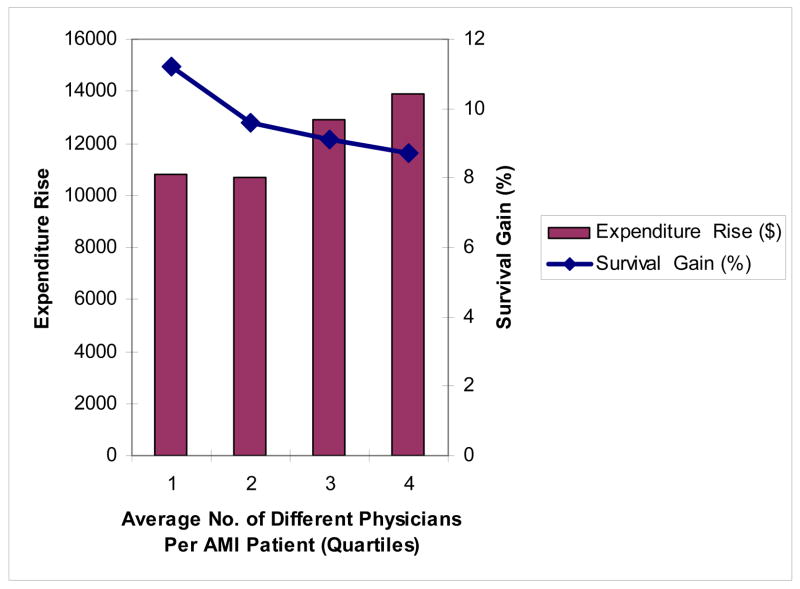
Exhibit 4 suggests large differences in regional productivity. Highly productive regions in the upper-left quadrant of Exhibit 4 are those that experienced rapid growth in survival but below-average growth in expenditures. Low productivity regions in the lower-right quadrant of Exhibit 4 experienced above-average cost increases with below-average survival gains. Can our two variables from 1994/95 – the quality index and the average number of different physicians – predict which regions ended up in the high or low productivity quadrants of Exhibit 4?
Regression results are shown in Exhibit 5A (for the quality index) and Exhibit 5B (for the specialization index). Exhibit 5A demonstrates a significantly greater improvement in survival among regions that had by 1994/95 adopted high quality treatment strategies for heart attacks, from 8.1 per 100 patients in the lowest quality regions to 12.5 per 100 patients in the highest quality regions (p = .01). Expenditure grew more slowly (by $2,262) in the highest quality regions compared to the lowest-quality regions, although the result was marginally significant (p = .06).
Exhibit 5.
Exhibit 5A: Association of Regional Quality of Care for AMI with Changes in Survival and Expenditures, 1986–2002
Exhibit 5B: Association of Average Number of Physicians Per AMI Patient (Quartiles) with Changes in Survival and Expenditures 1986–2002
Notes: Each bar shows the impact on the dependent variable (the change in dollar expenditures) of a shift from the 10th to the 90th percentile for each variable in a multiple regression framework. Ninety-five percent confidence intervals shown by whiskers.
Exhibit 5b shows the equivalent associations according to quartiles of the average number of different physicians visiting each patient. There are wide differences in the average number of physicians per patient, ranging from 4.8 different physicians per patient in Portland, OR to 9.2 different physicians in Philadelphia. Here the pattern is different; the improvement in survival is lower in regions with the highest number of different physicians (a survival gain of 8.7 per 100 AMI, versus 11.2 in the lowest areas, p = .06), while expenditure increases are substantially higher, by $3,331 (p = .001).
Note that Exhibit 5a controls for the average number of different physicians, while Exhibit 5b holds constant the quality index. The cumulative effect of varying both quality and the average number of physicians is large in magnitude and highly significant. The “highest productivity” regions (those with the best quality and lowest average number of physicians) are predicted to experience better survival growth (7.0 per 100) and lower cost increases ($5,593) than the “lowest productivity” regions.
Discussion
The last two decades have seen dramatic progress in the treatment of heart attacks among the elderly. Between 1986 and 2002, the average 1-year survival rate following AMI increased by nearly 10 per 100 elderly AMI patients at an estimated cost of less than $25,000 per life year saved. But underlying these numbers is tremendous heterogeneity across time and space: there was little improvement in survival after 1996 despite continued growth in costs, and there was substantial variation in survival gains across regions and over time, with regional gains that were (if anything) negatively related to costs.
These facts and others like them have generated a debate over the value of additional expenditures on medical care. On the one hand, aggregate trends in patient’s outcomes suggest that the technological innovations were “worth it.” In contrast, the apparent lack of any strong association between costs and patient outcomes or quality of care across regions suggests that aggressive cost-control policies may benefit society by eliminating unnecessary medical care being provided to patients in high-cost regions. 24
One approach to reconciling the two views in this debate is “flat of the curve”: Changes in patient outcomes over time reflect valuable technological change in medicine, but incremental spending across regions (beyond some minimum) provides little benefit or could even harm patients through medical errors or iatrogenic disease. 25 But “flat of the curve” explanations are not sufficient to explain the patterns we find. Simple overuse of unnecessary treatments cannot explain why high spending regions are less likely to provide inexpensive but effective treatments as found in the Fisher studies and Baicker and Chandra. Similarly, the “flat of the curve” hypothesis is not consistent with our findings that areas with lower gains in survival and higher cost growth lagged behind in adopting relatively low cost, non-invasive, treatments.
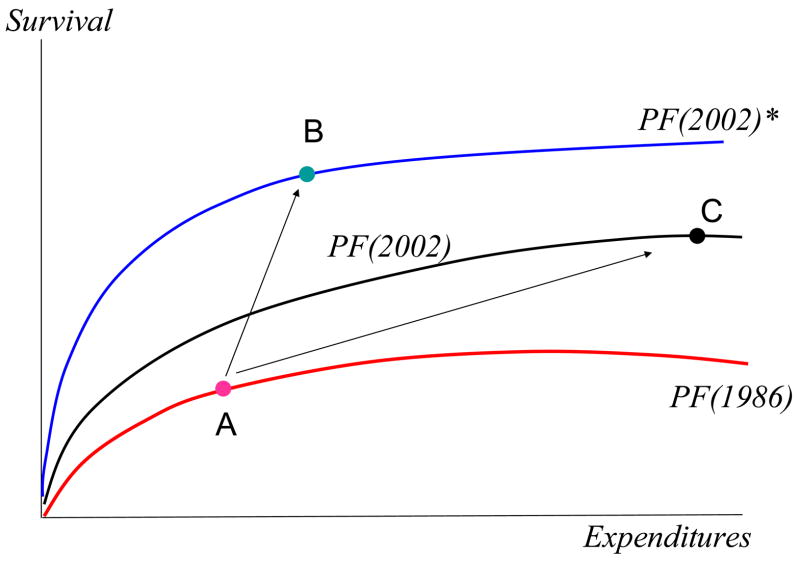
We suggest a different approach. Local regions where patients receive their care differ along numerous dimensions and thus each develop their own local constrained “production function” relating expenditures to health outcomes. 26 The bottom curve in Exhibit 6 shows a representative constrained production function in 1986. During the period 1986–2002, the constrained production function shifted to one of the upper curves showing the relationship between spending and survival in 2002. But the nature and magnitude of this upward shift may vary over time, just as it varies across regions. For example, in our hypothetical regions shown in Exhibit 6, the early adopter of effective care moved from A to B, the more efficient lower-cost equilibrium, while the region relying on multiple specialists but lagging in the adoption of effective care moved from A to C, the higher-cost less effective equilibrium. Note that this model reconciles the cross-sectional evidence: there is a negative correlation between spending and survival, as can be seen by comparing points B and C. But it also reconciles the time-series evidence: on average, everyone is better off, but the regional gains are not correlated with regional expenditure increases.
Exhibit 6. Technological Change in the Treatment of Heart Attacks: A Graphical Analysis.
A key assumption is that uneven diffusion of cost-effective innovations is a key factor driving differences in patient costs and outcomes. 27 Support for this view comes from Heidenreich and McClellan, who concluded that the vast majority of the increase in 30-day survival following AMI between 1975–95 was the consequence of low-cost treatments such as aspirin, β Blockers, ACE inhibitors, and thrombolytics. 28 Furthermore, both observational and clinical trial evidence suggests that use of these non-invasive treatments reduces the incremental benefit of more expensive treatments such as invasive surgery. 29 Therefore, adoption of such innovations may have caused a reduction in health care costs for many patients and thus resembled more closely those advances -- such as seen in computers -- that both reduce costs and improve quality.
The policy implications of this new approach are also quite different. In the “flat of the curve” approach, expenditures in high-cost regions could be reduced without an obvious adverse impact on outcomes. However, our new model does not preclude the possibility that sharp cutbacks in the low-efficiency high-cost regions could adversely affect quality of care. 30 Instead, policies should be directed towards improving productivity; restructuring hospital resources, improving the efficiency of physician treatment patterns, and accelerating the diffusion of highly effective treatments. Put more simply, the benefits of health care expenditures depend upon how one spends the money. 31 It is reassuring that our views are in concordance with those of Cutler and others, who also recognize the potential excess costs arising from marginally efficient modes of care and problems of coordination. 32
It is important to note that our new view does not apply solely to “low tech” effective treatments such as aspirin and beta blockers, but applies equally to any highly effective treatment, whether high-tech or low-tech. Indeed, we have found in earlier research that regional rates of high-tech surgical treatments such as angioplasty and back surgery are uncorrelated with per capita Medicare expenditures. 33 This paper has not examined diffusion in surgical interventions, but preliminary results suggest that this was not the primary cause of expenditure growth during this period.
The benefits of health care technology are often substantial. However, as health care costs continue to rise, squeezing consumers, producers, and the federal budget alike, principles of accountability, that each incremental dollar should provide something of real value to patients, become increasingly important as a guiding principle. That some regions were able to implement technological innovations at remarkably low cost is a reminder that waste and inefficiency is not an inevitable byproduct of technological growth. Thus efforts to develop measures of quality and efficiency that can encourage hospitals or provider groups to adopt low-cost highly effective care, while discouraging incremental spending with no apparent benefits, may allow us to keep the golden goose of technological progress alive and well nourished.
Acknowledgments
We are grateful to NIA Grant No. PO1-AG19783 for financial support, to Amitabh Chandra, David Cutler, Mark McClellan, John E. Wennberg, and two anonymous referees for helpful and constructive comments, and to Weiping Zhou and Daniel Gottlieb for expert research assistance.
References
- 1.See Cutler DM, McClellan M. Is Technological Change in Medicine Worth It? Health Affairs. 2001 Sept/Oct;:11–29. doi: 10.1377/hlthaff.20.5.11.Cutler DM. Your Money or Your Life: Strong Medicine for America's Health Care System. New York: Oxford University Press; 2004. The pioneering study is Cutler DM, et al. Are Medical Prices Declining? Evidence from Heart Attack Treatments. Quarterly Journal of Economics. 1998 November;113(4):991–1024.
- 2.The typical hurdle for cost-effectiveness studies is $50,000 to $100,000 per life year, with some authors arguing that society places even greater value on life-years. See Hirth RA, Chernew ME, Fendrick M. What is the Price of Life and Why Doesn’t It Increase at the Rate of Inflation? Archives of Internal Medicine. 2003 July 28;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637.
- 3.Also see Berndt ER, et al. The Medical Treatment of Depression, 1991–1996: Productive Inefficiency, Expected Outcome Variations and Price Indexes. Journal of Health Economics. 2002;21(3):373–396. doi: 10.1016/s0167-6296(01)00132-1.Lichtenberg F. The Expanding Pharmaceutical Arsenal in the War on Cancer. In: Murphey Kevin, Robert Topel., editors. Measuring the Gains from Medical Research: An Economic Approach. Chicago: University of Chicago Press; 2003. NBER Working Paper No. 10328 (February 2004), and papers.
- 4.Fisher ES, et al. The Implications of Regional Variations in Medicare Spending. Part 1: The Content, Quality, and Accessibility of Care. Annals of Internal Medicine. 2003 February 18;138(4):283–287. doi: 10.7326/0003-4819-138-4-200302180-00006. and The Implications of Regional Variations in Medicare Spending. Part 2: Health Outcomes and Satisfaction With Care” Annals of Internal Medicine 138 No. 4 (February 18, 2003): 288–299. For a formal instrumental variables analysis, see Jonathan Skinner, Fisher Elliott, Wennberg John. The Efficiency of Medicare. In: Wise D, editor. Analyses in the Economics of Aging. Chicago: University of Chicago Press and NBER; 2005. p. 157.
- 5.Amitabh Chandra, Baicker Katherine. Medicare Spending and the Quality of Care Received by Medicare Beneficiaries. Health Affairs. 2004 April 7;:W4:184–W4:197. doi: 10.1377/hlthaff.w4.184. web exclusive. [DOI] [PubMed] [Google Scholar]
- 6.An AMI corresponded a hospital admission with a primary diagnosis code of 410x0 or 410x1 where x ranges from 0 to 9.
- 7.Other exclusions included the inability to match to a regional category, and if patients were enrolled in an HMO at the time of the heart attack.
- 8.Council of Economic Advisors, U.S. Government, Economic Report of the President 2005.
- 9.A linear rather than logistic approach for mortality was used to facilitate the (linear) decomposition of overall changes into regional-specific changes.
- 10.Wennberg JE, Cooper MM, editors. The Dartmouth Atlas of Healthcare. Hanover NH and Chicago: American Hospital Association; 1999. [Google Scholar]
- 11.Second International Study of Infarct Survival (ISIS-2) Randomized Trial of Intravenous Streptokinase, oral aspirin, Both or Neither Among 17,187 Cases of Suspected Acute Myocardial Infarction: ISIS-2. Lancet. 1988 August 13;332(8607):349–360. [PubMed] [Google Scholar]
- 12.See Yusuf S, et al. Beta Blockage During and After Myocardial Infarction: An Overview of the Randomized Trials. Progress in Cardiovascular Disease. 1985 March/April;27:335–71. doi: 10.1016/s0033-0620(85)80003-7.
- 13.Jencks SF, Huff ED, Cuerdon T. Change in the Quality of Care Delivered to Medicare Beneficiaries, 1998–99 to 2000–2001. JAMA. 2003 January 15;289(3):305–312. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- 14.International Society and Federation of Cardiology and World Health Organization Task Force on Myocardial Reperfusion. Reperfusion in Acute Myocardial Infarction. Circulation. 1994 October 1;90(4):2091–2102. doi: 10.1161/01.cir.90.4.2091. [DOI] [PubMed] [Google Scholar]
- 15.Several HRRs were excluded from the analysis because of insufficient sample size in the quality indicators. See Birkmeyer J, Wennberg D, editors. Dartmouth Atlas of Cardiovascular Healthcare. Hanover, NH: Dartmouth Medical School; 2000.
- 16.This follows the terminology in Rogers EM. Diffusion of Innovations. 4. New York: The Free Press; 1995.
- 17.Murphy K, Becker G. The Division of Labor, Coordination Costs, and Knowledge. Quarterly Journal of Economics. 1992 November;107(4):1137–60., and Baicker K, Chandra A. The Productivity of Physician Specialization: Evidence from the Medicare Program. American Economic Review. 2004 May;94(2):357–361. doi: 10.1257/0002828041301461.
- 18.Diane Payne. I Shouldn’t Have Had to Beg for a Prognosis. Newsweek. 2005 August 22;:16. [Google Scholar]
- 19.One concern with our measure of network effects is that the average number of different physicians simply reflects unmeasured confounding factors; sicker patients require more physicians. However, there was essentially no correlation at the regional level between an index of AMI severity – predicted mortality – and the average number of different physicians(ρ = 0.06, p = .33).
- 20.A large and statistically significant negative correlation obtains even when the high expenditure regions (such as the rightmost three regions in Exhibit 1) are excluded.
- 21.We are grateful to David Cutler for providing these data.
- 22.See Ash S, et al. Using Claims Data to Examine Mortality Trends Following Hospitalization for Heart Attack in Medicare – Access and Quality. Health Services Research. 2003 October;38(5):1253–62. doi: 10.1111/1475-6773.00175. Alternatively, it could be that during the later 1990s, the rising fraction of Medicare enrollees in HMOs resulted in fee-for-service patients who were increasingly sicker. However, one study shows little evidence that after adjusting for comorbidities (as we do), HMO patients were more likely to survive AMI; see Luft HS. Variations in Patterns of Care and Outcomes After Acute Myocardial Infarction for Medicare Beneficiaries in Fee-For-Service and HMO Settings. Health Services Research. 2003 August;38(4):1065–1079. doi: 10.1111/1475-6773.00163.
- 23.See Heidenreich PA, McClellan M. Trends in Treatment and Outcomes for Acute Myocardial Infarction: 1975–1995. American Journal of Medicine. 2001 February 15;110(3):165–74. doi: 10.1016/s0002-9343(00)00712-9. A meta-analysis of stents did not find any significant impact on mortality Zhu M, et al. Primary Stent Implantation Compared with Primary Balloon Angioplasty for Acute Myocardial Infarction: A Meta-analysis of Randomized Clinical Trials. The American Journal of Cardiology. 2001 August 1;88(3):297–301. doi: 10.1016/s0002-9149(01)01645-9.
- 24.See Footnote 4.
- 25.For example see Cutler DM. Walking the Tightrope on Medicare Reform. Journal of Economic Perspectives. 2000 Spring;14(2):45–56. doi: 10.1257/jep.14.2.45.
- 26.The production functions are constrained because lack of knowledge and skills, or poor organizational structure, may prevent health care systems from attaining the efficient (and perhaps hypothetical) “best practice” production function.
- 27.Berwick D. Disseminating Innovations in Health Care. JAMA. 2003 April 16;289(15):1969–1975. doi: 10.1001/jama.289.15.1969. There is a parallel literature in macroeconomics that stresses the role of the diffusion of innovations in long-term differences in country income and growth. See Parente SL, Prescott EC. Barriers to Technology Adoption and Development. The Journal of Political Economy. 1994 April;102(2):298–321. and Skinner J, Staiger D. Technology Adoption from Hybrid Corn to Beta Blockers. NBER Working Paper No 11251. 2005 April;
- 28.Heidenreich PA, McClellan M. Trends in Treatment and Outcomes for Acute Myocardial Infarction: 1975–1995. doi: 10.1016/s0002-9343(00)00712-9. [DOI] [PubMed] [Google Scholar]
- 29.Stukel T, Lucas L, Wennberg DE. Long-Term Outcomes of Regional Variations in Intensity of Invasive vs. Medical Management of Medicare Patients with Acute Myocardial Infarction. JAMA. 2005 March 16;293(11):1329–1337. doi: 10.1001/jama.293.11.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]; de Winter Robbert J, et al. Early Invasive versus Selectively Invasive Management for Acute Coronary Syndromes. New England Journal of Medicine. 2005 September 15;353(11):1095–1104. doi: 10.1056/NEJMoa044259. [DOI] [PubMed] [Google Scholar]
- 30.Baicker K, Staiger D. Fiscal Shenanigans, Targeted Federal Health Care Funds, and Patient Mortality. Quarterly Journal of Economics. 2005 January;120(1):345–86. doi: 10.1162/0033553053327461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowd B. Perspective: The Problem of Multiple Margins. Health Affairs Web Exclusive. 2004 October 7;:Var112–Var116. doi: 10.1377/hlthaff.var.112. [DOI] [PubMed] [Google Scholar]
- 32.Cutler DM, Huckman RS, Landrum MB. NBER Working Paper No. 10489. May, 2004. The Role of Information in Medical Markets: An Analysis of Publicly Reported Outcomes in Cardiac Surgery.Cutler DM, Wennberg JE, et al. Use of Hospitals, Physician Visits, and Hospice Care During the Last Six Months of Life among Cohorts Loyal to Highly Respected Hospitals in the United States. British Medical Journal. 2004 March 13;328(7440):607–611. doi: 10.1136/bmj.328.7440.607. Your Money or Your Life. and Wennberg JE, et al. Use of Medicare Claims Data to Monitor Provider-Specific Performance among Patients with Severe Chronic Illness. Health Affairs web exclusive. 2004 October 7;:Var5–Var18. doi: 10.1377/hlthaff.var.5.
- 33.Wennberg JE, Fisher ES, Skinner J. Geography and the Debate Over Medicare Reform. Health Affairs Web Exclusive. 2002 13 February;:W96–W114. doi: 10.1377/hlthaff.w2.96. [DOI] [PubMed] [Google Scholar]