Short Communication
1H,1H,2H,2H,3H,3H-perfluoroundecanol- and 1H,1H,2H,2H-perfluorodecanethiol-displaced 2,4-dichloro-1,3,5-triazines 1 and 2 are synthesized and used as nucleophile scavengers to remove thiols and amines from solution-phase reactions. Two active sites on the triazine ring are able to remove two equivalents of nucleophiles. The purification of reaction mixtures is accomplished by plate-to-plate fluorous solid-phase extraction or automated solid-phase extraction on the RapidTrace system.
Introduction
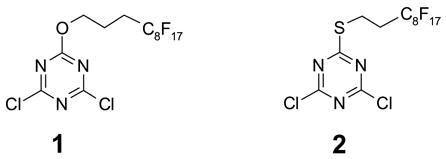
2,4,6-Trichloro-1,3,5-triazine (cyanuric chloride) and its derivatives have been widely used as chlorination and condensation agents in organic synthesis, as complexation agents in analytical chemistry, as multi-step redox agents in electrochemistry, as pesticides and herbicides in agricultural chemistry, as templates in crystal engineering, as scaffolds in combinatorial chemistry, and as building blocks for peptidomimetics [1]. 2-Chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) is a well-known amide coupling agent [2]. The polymer-supported [3] and the fluorous [4,5] version of CDMTs have been developed for parallel and combinatorial syntheses. 2,4-Dichloro-6-methoxy-1,3,5-triazines (DCT) [6] and its solid-supported analogs [7] have also been employed as amide coupling agents as well as nucleophile scavengers. We recently synthesized fluorous alkoxy- and alkylthio-attached DCT compounds 1 and 2, and explored their utility in fluorous amide coupling reactions [8]. We report here the use of F-DCTs as nucleophile scavengers for fluorous solution-phase synthesis [9,10]. Since F-DCTs have two active electrophiles, we expected they can be used to remove two equivalents of nucleophiles from the reaction mixtures.
Results
The preparation of 1 was accomplished by reaction of 1.0 equiv of 1H,1H,2H,2H,3H,3H-perfluoroundecanol with 1.05 equiv of cyanuric chloride and 1.5 equiv of 2,4,6-collidine in benzotrifluoride (BTF) at room temperature for 10 h (Scheme 1, A). After removal of collidine salt by filtration, the concentrated crude product was passed through a plug of silica gel and eluted with 10% EtOAc-hexane. The crude product was further purified by recrystalization in hexane or by normal silica gel chromatography. F-DCT 2 was synthesized by reaction of cyanuric chloride with 1H,1H,2H,2H-perfluorodecanethiol. The reaction was performed using 1.5 equiv of cyanuric chloride at −30 °C to minimize the formation of dithiolation byproduct (Scheme 1, B). Both F-DCTs 1 and 2 are white solids, while compound 2 was found more stable for room temperature storage.
Scheme 1.
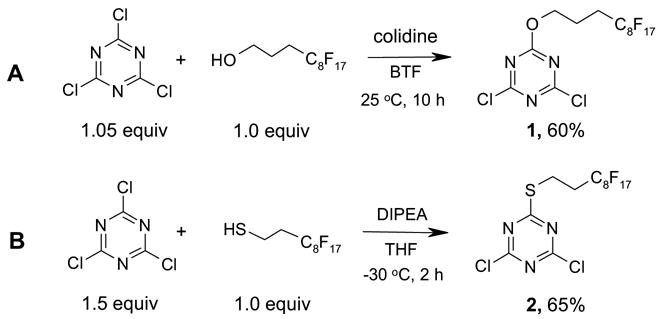
Synthesis of F-DCTs 1 and 2
With F-DCTs 1 and 2 in hand, we first studied their reactions with thiols (Scheme 2, A). A THF solution containing 0.2 mmol of DIPEA, 0.075 mmol of F-DCT 1 was reacted with 0.1 mmol of 4-methoxybenzyl thiol. After 1 h at room temperature, LCMS analysis with mass ion extraction and UV detection showed no thiol but monothio- and dithio-substituted triazines 3 and 4 in the reaction mixture. Similarly, monoamino- and diamino-substituted triazines 5 and 6 were detected from the reaction of 0.075 mmol of 1 with 0.1 mmol of tetrahydroisoquinoline (Scheme 2, B). The results suggest that both chlorines of the F-DCTs are reactive enough for thiol and amine scavenging reactions. The reactions of F-DCT 2 with 4-methoxybenzyl thiol or tetrahydroisoquinoline gave similar results; no noticeable differences were observed between F-DCTs 1 and 2.
Scheme 2.
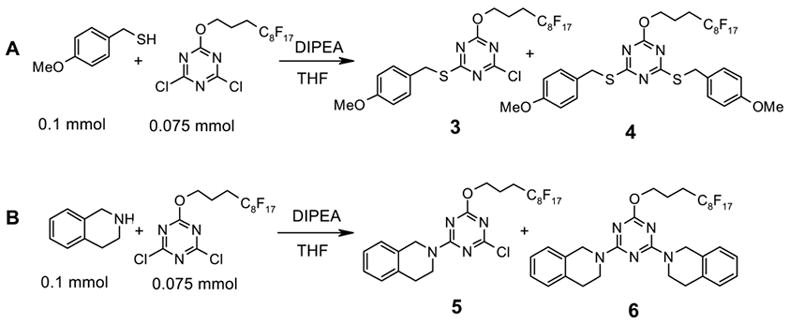
Reactions of F-DCT 1 with thiol and amine
Encouraged by the initial results, we then used F-DCT 2 as a scavenger in an S-alkylation reaction to remove excess thiols (Scheme 3). Thus, a mixture of 0.1 mmol of a bromide, 0.15 mmol of 4-methoxybenzyl thiol, and 0.3 mmol DIPEA in 0.5 mL of CH2Cl2 was shaken at room temperature for 1 h before 0.05 mmol of F-DCT 2 in CH2Cl2 was added. The reaction mixture was shaken for another 1 h and then 0.6 mmol equiv of MP-CO3 [11] was added to free the DIPEA salt. After filtration, the concentrated reaction mixture was dissolved in 0.6 mL of DMF and then loaded onto a 2 g FluoroFlash cartridge for fluorous solid-phase extraction (F-SPE) [12]. The cartridge was eluted with 6 mL of 8:2 MeOH/H2O to elute the S-alkylation product, while the fluorous components remained on the cartridge. TheMeOH/H2O fraction was concentrated to afford product 7 in 65% yield and 91% purity by LCMS analysis with UV254. From a control experiment without using F-DCT 2 as scavenger, a significant amount of 4-methoxybenzyl thiol was detected from the S-alkylation product. This result indicates that the thiol is not acidic enough to be trapped by MP-CO3 and separated from the alkylated product.
Scheme 3.
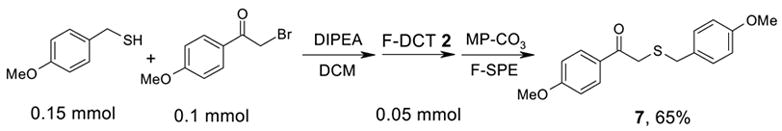
S-Alkylation reaction with F-DCT 2 as thiol scavenger
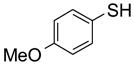
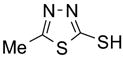
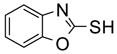
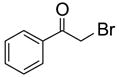
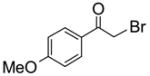
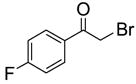
To study the reaction scope and evaluate the feasibility of solution-phase parallel synthesis, eighteen reactions were conducted using three activated halides and six thiols including a thiolphenol and two heteroaromatic thiols. The reaction mixtures were purified by the newly developed plate-to-plate F-SPE [5]. Results summarized in Table 1 indicate that the yields of S-alkylation are in the range of 63–96%, fifteen out of eighteen products have purities greater than 90%.
Table 1.
S-Alkylation using F-DCT 2 as thiol scavenger
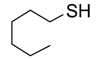
|
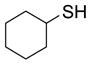
|
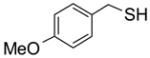
|
|
|
|
|
|
91%(92%)* | 88%(95%) | 85%(94%) | 92%(94%) | 80%(92%) | 63%(99%) |
|
70%(91%) | 78%(81%) | 67%(87%) | 86%(93%) | 71%(94%) | 87%(99%) |
|
88%(97%) | 96%(91%) | 83%(87%) | 86%(93%) | 63%(96%) | 76%(95%) |
yield% (purity%), purities were determined by LCMS with UV254 detection
We also employed F-DCT 1 as amine scavenger in the reactions of isocyanates and amines (Scheme 4). A general procedure was developed as such: a mixture of 0.1 mmol of isocyanate and 0.15 mmol of amine in 0.5 mL of THF was shaken at room temperature for 1 h before 0.05 mmol of F-DCT 1 and 0.3 mmol of DIPEA was added. The reaction mixture was shaken for 1 h and then 0.6 mmol equiv MP-CO3 was added to free the DIPEA salt. After filtration, the concentrated reaction mixture was dissolved in 0.6 mL of DMF and then loaded onto a 2 g FluoroFlash cartridge for F-SPE. The cartridge was eluted with 6 mL of 8:2 MeOH/H2O to elute the urea product, while the fluorous components were remained on the cartridge. The MeOH/H2O fraction was concentrated to afford product 8 in 62% yield and 96% purity by LCMS analysis with UV254. In separate experiments, we found that F-DCT did not react with ureas generated from isocyanates and primary amines. However, it reacted with thioureas generated from thioisocyanates and amines.
Scheme 4.
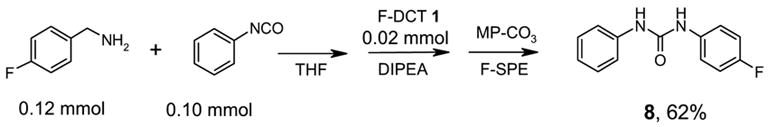
Urea formation reaction with F-DCT 1 as amine scavenger
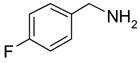
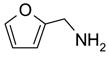
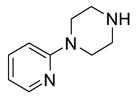
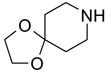
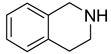
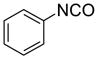
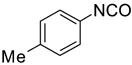
Eighteen reactions were conducted in parallel using three isocyanates and six amines followed by amine scavenging with F-DCT 1. Instead of performing plate-to-plate SPE, we conducted automated SPE on a RapidTrace SPE station [13] equipped with 1.5 g FluoroFlash cartridges. Results summarized in Table 2 show that the yields of ureas are in the range of 48–96%, thirteen out of eighteen products have purities greater than 90%.
Table 2.
Urea formation reactions using F-DCT 1 as amine scavenger
| BuNH2 |
|
|
|
|
|
|
|
47%(93%)* | 63%(94%) | 64%(94%) | 89%(96%) | 92%(90%) | 96%(80%) |

|
67%(96%) | 58%(96%) | 52%(96%) | 63%(95%) | 57%(94%) | 48%(97%) |
|
57%(77%) | 77%(98%) | 87%(90%) | 96%(86%) | 93%(80%) | 79%(87%) |
yield% (purity%), purities were determined by LCMS with UV254 detection
In summary, we have synthesized two fluorous DCT compounds and demonstrated their utilities as thiol and amine scavengers. Compared to their polymer- or silica gel-supported analogs, F-DCTs have good solution-phase reaction kinetics [14]. Reactions go faster and do not require large excess amount of scavengers to complete the reactions. Two active sites of F-DCTs are capable to remove up to two equiv of nucleophiles form the reaction mixtures.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences SBIR Grants (2R44GM062717-02 and 2R44GM067326-02).
References and Notes
- 1.Giacomelli G, Porcheddu A, DeLuca L. Curr Org Chem. 2004;8:1497–1519. and references cited therein. [Google Scholar]
- 2.Selected references: Bandgar BP, Pandit SS. Tetrahedron Lett. 2003;44:3855–3858.De Luca L, Giacomelli G, Taddei M. J Org Chem. 2001;66:2534–2537. doi: 10.1021/jo015524b.Kunishima M, Yoshimura K, Morigaki H, Kawamata R, Terao K, Tani S. J Am Chem Soc. 2001;123:10760–10761. doi: 10.1021/ja011660m.Pontikis R, Dolle V, Guillaumel J, Dechaux E, Note R, Nguyen CH, Legraverend M, Bisagni E, Aubertin A-M, Grierson DS, Monneret C. J Med Chem. 2000;43:1927–1939. doi: 10.1021/jm991125l.Kamiñska JE, Kamiñski ZJ, Góra J. Synthesis. 1999:593–596.
- 3.a) Xia M, Wang Y. Syn Commun. 2003;33:403–408. [Google Scholar]; b) Bork JT, Lee JW, Chang YT. Tetrahedron Lett. 2003;44:6141–6144. [Google Scholar]
- 4.Markowicz MW, Dembinski R. Synthesis. 2004:80–86. [Google Scholar]
- 5.Zhang W, Lu Y, Nagashima T. J Comb Chem. 2005;7:893–897. doi: 10.1021/cc050061z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminski ZJ. Biopolymers (Pept Sci) 2000;55:140–164. doi: 10.1002/1097-0282(2000)55:2<140::AID-BIP40>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.a) Falchi A, Taddei M. Org Lett. 1999;1:1355–1357. [Google Scholar]; b) Falchi A, Taddei M. Org Lett. 2000;2:3429–3431. doi: 10.1021/ol0002222. [DOI] [PubMed] [Google Scholar]; c) Marsh A, Carlisle SJ, Smith SC. Tetrahedron Lett. 2001;42:493–496. [Google Scholar]
- 8.Zhang W, Lu Y. QSAR Comb Sci. 2006 doi: 10.1002/qsar.200640041. in this issue (need update once paper # is assigned) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selected reviews on fluorous synthesis: Curran DP. Aldrichmica Acta. 2006;39:3–9.Gladysz JA, Curran DP, Horvath IT, editors. Handbook of Fluorous Chemistry. Wiley-VCH; Weinheim: 2004. Zhang W. Chem Rev. 2004;104:2531–2556. doi: 10.1021/cr030600r.Zhang W. Curr Opin Drug Disc Dev. 2004;7:784–797.Zhang W. Tetrahedron. 2003;59:4475–4489.Horvath IT. Acc Chem Res. 1998;31:641–650.Studer A, Hadida S, Ferritto SY, Kim PY, Jeger P, Wipf P, Curran DP. Science. 1997;275:823–82. doi: 10.1126/science.275.5301.823.
- 10.a) Lindsley CW, Leister WH. Fluorous scavengers. In: Gladysz JA, Curran DP, Horvath IT, editors. Handbook of Fluorous Chemistry. Wiley-VCH; Weinheim: 2004. pp. 236–246. [Google Scholar]; b) Lindsley CW, Bogusky MJ, Leister WH, McClain RT, Robinson RG, Barnett SF, Defeo-Jones D, Hartman GD. Tetrahedron Lett. 2005;46:2779–2782. [Google Scholar]; c) Zhang AS, Elmore CS, Egan MA, Melillo DG, Dean DC. J Label Compd Radiopharm. 2005;48:203–208. [Google Scholar]; d) Zhang W, Chen CHT, Nagashima T. Tetrahedron Lett. 2003;44:2065–2068. [Google Scholar]; e) Werner S, Curran DP. Org Lett. 2003;5:3293–3296. doi: 10.1021/ol035214a. [DOI] [PubMed] [Google Scholar]; f) Lindsley CW, Zhao Z, Leister WH. Tetrahedron Lett. 2002;43:4225–4228. [Google Scholar]; g) Zhang W, Curran DP, Chen CHT. Tetrahedron. 2002;58:3871–3875. [Google Scholar]
- 11.MP-carbonate is a product of Argonaut Technologies. www.argotech.com.
- 12.FluoroFlash SPE cartridges are available from Fluorous Technologies, Inc., www.fluorous.com. General information for the use of FluoroFlash cartridges, see: http://fluorous.com/download/FTI_AppNote_F-SPE.pdf
- 13.RapidTrace system is available from Caliper Life Sciences, www.caliperls.com
- 14.Chen CHT, Zhang W. Mol Diversity. 2005;9:353–359. doi: 10.1007/s11030-005-8104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


